Role of the serine-rich surface glycoprotein Srr1 of Streptococcus agalactiae in the pathogenesis of infective endocarditis
- PMID: 23717569
- PMCID: PMC3662765
- DOI: 10.1371/journal.pone.0064204
Role of the serine-rich surface glycoprotein Srr1 of Streptococcus agalactiae in the pathogenesis of infective endocarditis
Abstract
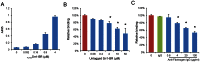
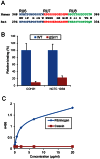
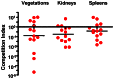
The binding of bacteria to fibrinogen and platelets are important events in the pathogenesis of infective endocarditis. Srr1 is a serine-rich repeat glycoprotein of Streptococcus agalactiae that binds directly to the Aα chain of human fibrinogen. To assess the impact of Srr1 on the pathogenesis of endocarditis due to S. agalactiae, we first examined the binding of this organism to immobilized human platelets. Strains expressing Srr1 had significantly higher levels of binding to human platelets in vitro, as compared with isogenic Δsrr1 mutants. In addition, platelet binding was inhibited by pretreatment with anti-fibrinogen IgG or purified Srr1 binding region. To assess the contribution of Srr1 to pathogenicity, we compared the relative virulence of S. agalactiae NCTC 10/84 strain and its Δsrr1 mutant in a rat model of endocarditis, where animals were co-infected with the WT and the mutant strains at a 1:1 ratio. At 72 h post-infection, bacterial densities (CFU/g) of the WT strain within vegetations, kidneys, and spleens were significantly higher, as compared with the Δsrr1 mutant. These results indicate that Srr1 contributes to the pathogenesis of endocarditis due to S. agalactiae, at least in part through its role in fibrinogen-mediated platelet binding.
Conflict of interest statement
Figures

References
-
- Sunkara B, Bheemreddy S, Lorber B, Lephart PR, Hayakawa K, et al. (2012) Group B Streptococcus infections in non-pregnant adults: the role of immunosuppression. Int J Infect Dis 16: e182–186. - PubMed
-
- Farley MM (2001) Group B streptococcal disease in nonpregnant adults. Clin Infect Dis 33: 556–561. - PubMed
-
- Munoz P, Llancaqueo A, Rodriguez-Creixems M, Pelaez T, Martin L, et al. (1997) Group B streptococcus bacteremia in nonpregnant adults. Arch Intern Med 157: 213–216. - PubMed
-
- Siciliano RF, Cais DP, Navarro RC, Strabelli TM (2010) Acute Streptococcus agalactiae endocarditis: outcomes of early surgical treatment. Heart Lung 39: 331–334. - PubMed
-
- Ivanova Georgieva R, Garcia Lopez MV, Ruiz-Morales J, Martinez-Marcos FJ, Lomas JM, et al. (2010) Streptococcus agalactiae left-sided infective endocarditis. Analysis of 27 cases from a multicentric cohort. J Infect 61: 54–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

