The combination of novel targeted molecular agents and radiation in the treatment of pediatric gliomas
- PMID: 23717811
- PMCID: PMC3650671
- DOI: 10.3389/fonc.2013.00110
The combination of novel targeted molecular agents and radiation in the treatment of pediatric gliomas
Abstract
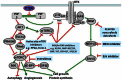
Brain tumors are the most common solid pediatric malignancy. For high-grade, recurrent, or refractory pediatric brain tumors, radiation therapy (XRT) is an integral treatment modality. In the era of personalized cancer therapy, molecularly targeted agents have been designed to inhibit pathways critical to tumorigenesis. Our evolving knowledge of genetic aberrations in pediatric gliomas is being exploited with the use of specific targeted inhibitors. These agents are additionally being combined with XRT to increase the efficacy and duration of local control. In this review, we discuss novel agents targeting three different pathways in gliomas, and their potential combination with XRT. BRAF is a serine/threonine kinase in the RAS/RAF/MAPK kinase pathway, which is integral to cellular division, survival, and metabolism. Two-thirds of pilocytic astrocytomas, a low-grade pediatric glioma, contain a translocation within the BRAF gene called KIAA1549:BRAF that causes an overactivation of the MEK/MAPK signaling cascade. In vitro and in vivo data support the use of MEK or mammalian target of rapamycin (mTOR) inhibitors in low-grade gliomas expressing this translocation. Additionally, 15-20% of high-grade pediatric gliomas express BRAF V600E, an activating mutation of the BRAF gene. Pre-clinical in vivo and in vitro data in BRAF V600E gliomas demonstrate dramatic cooperation between XRT and small molecule inhibitors of BRAF V600E. Another major signaling cascade that plays a role in pediatric glioma pathogenesis is the PI3-kinase (PI3K)/mTOR pathway, known to be upregulated in the majority of high- and low-grade pediatric gliomas. Dual PI3K/mTOR inhibitors are in clinical trials for adult high-grade gliomas and are poised to enter studies of pediatric tumors. Finally, many brain tumors express potent stimulators of angiogenesis that render them refractory to treatment. An analog of thalidomide, CC-5103 increases the secretion of critical cytokines of the tumor microenvironment, including IL-2, IFN-γ, TNF-α, and IL-10, and is currently being evaluated in clinical trials for the treatment of recurrent or refractory pediatric central nervous system tumors. In summary, several targeted inhibitors with radiation are currently under investigation in both translational bench research and early clinical trials. This review article summarizes the molecular rationale for, and the pre-clinical data supporting the combinations of these targeted agents with other anti-cancer agents and XRT in pediatric gliomas. In many cases, parallels are drawn to molecular mechanisms and targeted inhibitors of adult gliomas. We additionally discuss the potential mechanisms underlying the efficacy of these agents.
Keywords: BRAFV600E; PI3K/AKT/mTOR; angiogenesis inhibitors; bevacizumab; lenalidomide; low-grade glioma; pediatric gliomas.
Figures
Similar articles
-
BRAF Status in Personalizing Treatment Approaches for Pediatric Gliomas.Clin Cancer Res. 2016 Nov 1;22(21):5312-5321. doi: 10.1158/1078-0432.CCR-15-1101. Epub 2016 May 23. Clin Cancer Res. 2016. PMID: 27217440 Free PMC article.
-
Dual Inhibition of MAPK and TORC1 Signaling Retards Development of Radiation Resistance in Pediatric BRAFV600E Glioma Models.Neuro Oncol. 2025 Mar 14:noaf068. doi: 10.1093/neuonc/noaf068. Online ahead of print. Neuro Oncol. 2025. PMID: 40083135
-
BRAF and MEK Targeted Therapies in Pediatric Central Nervous System Tumors.Cancers (Basel). 2022 Aug 31;14(17):4264. doi: 10.3390/cancers14174264. Cancers (Basel). 2022. PMID: 36077798 Free PMC article. Review.
-
Resistance mechanisms in BRAFV600E paediatric high-grade glioma and current therapeutic approaches.Front Oncol. 2022 Dec 13;12:1031378. doi: 10.3389/fonc.2022.1031378. eCollection 2022. Front Oncol. 2022. PMID: 36582791 Free PMC article. Review.
-
Implications of BRAF V600E mutation in gliomas: Molecular considerations, prognostic value and treatment evolution.Front Oncol. 2023 Jan 4;12:1067252. doi: 10.3389/fonc.2022.1067252. eCollection 2022. Front Oncol. 2023. PMID: 36686797 Free PMC article. Review.
Cited by
-
Paediatric gliomas: diagnosis, molecular biology and management.Ann Transl Med. 2018 Jun;6(12):251. doi: 10.21037/atm.2018.05.11. Ann Transl Med. 2018. PMID: 30069453 Free PMC article. Review.
-
Canonical and Non-Canonical Roles of PFKFB3 in Brain Tumors.Cells. 2021 Oct 27;10(11):2913. doi: 10.3390/cells10112913. Cells. 2021. PMID: 34831136 Free PMC article. Review.
-
Molecular characteristics of pediatric high-grade gliomas.CNS Oncol. 2014 Nov;3(6):433-43. doi: 10.2217/cns.14.43. CNS Oncol. 2014. PMID: 25438814 Free PMC article. Review.
-
Exploiting molecular biology for diagnosis and targeted management of pediatric low-grade gliomas.Future Oncol. 2016 Jun;12(12):1493-506. doi: 10.2217/fon-2016-0039. Epub 2016 Apr 13. Future Oncol. 2016. PMID: 27072750 Free PMC article. Review.
-
High rate of concurrent BRAF-KIAA1549 gene fusion and 1p deletion in disseminated oligodendroglioma-like leptomeningeal neoplasms (DOLN).Acta Neuropathol. 2015 Apr;129(4):609-610. doi: 10.1007/s00401-015-1400-9. Epub 2015 Feb 27. Acta Neuropathol. 2015. PMID: 25720745 Free PMC article. No abstract available.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous