Effect of nitric oxide on naphthoquinone toxicity in endothelial cells: role of bioenergetic dysfunction and poly (ADP-ribose) polymerase activation
- PMID: 23718265
- PMCID: PMC4028014
- DOI: 10.1021/bi400342t
Effect of nitric oxide on naphthoquinone toxicity in endothelial cells: role of bioenergetic dysfunction and poly (ADP-ribose) polymerase activation
Abstract
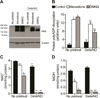
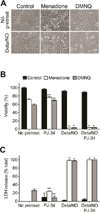
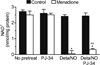
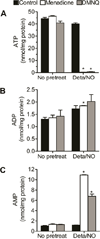
When produced at physiological levels, reactive oxygen species (ROS) can act as signaling molecules to regulate normal vascular function. Produced under pathological conditions, ROS can contribute to the oxidative damage of cellular components (e.g., DNA and proteins) and trigger cell death. Moreover, the reaction of superoxide with nitric oxide (NO) produces the strong oxidant peroxynitrite and decreases NO bioavailability, both of which may contribute to activation of cell death pathways. The effects of ROS generated from the 1,4-naphthoquinones alone and in combination with NO on the activation status of poly(ADP-ribose) polymerase (PARP) and cell viability were examined. Treatment with redox cycling quinones activates PARP, and this stimulatory effect is attenuated in the presence of NO. Mitochondria play a central role in cell death signaling pathways and are a target of oxidants. We show that simultaneous exposure of endothelial cells to NO and ROS results in mitochondrial dysfunction, ATP and NAD(+) depletion, and cell death. Alone, NO and ROS have only minor effects on cellular bioenergetics. Further, PARP inhibition does not attenuate reduced cell viability or mitochondrial dysfunction. These results show that concomitant exposure to NO and ROS impairs energy metabolism and triggers PARP-independent cell death. While superoxide-mediated PARP activation is attenuated in the presence of NO, PARP inhibition does not modify the loss of mitochondrial function or adenine and pyridine nucleotide pools and subsequent bioenergetic dysfunction. These findings suggest that the mechanisms by which ROS and NO induce endothelial cell death are closely linked to the maintenance of mitochondrial function and not overactivation of PARP.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
Similar articles
-
Purines inhibit poly(ADP-ribose) polymerase activation and modulate oxidant-induced cell death.FASEB J. 2001 Jan;15(1):99-107. doi: 10.1096/fj.00-0299com. FASEB J. 2001. PMID: 11149897
-
Poly(ADP-ribose) Polymerase (PARP) and PARP Inhibitors: Mechanisms of Action and Role in Cardiovascular Disorders.Cardiovasc Toxicol. 2018 Dec;18(6):493-506. doi: 10.1007/s12012-018-9462-2. Cardiovasc Toxicol. 2018. PMID: 29968072 Review.
-
Reactive oxygen injury to cultured pulmonary artery endothelial cells: mediation by poly(ADP-ribose) polymerase activation causing NAD depletion and altered energy balance.Arch Biochem Biophys. 1991 May 1;286(2):353-63. doi: 10.1016/0003-9861(91)90051-j. Arch Biochem Biophys. 1991. PMID: 1654786
-
Poly(ADP-ribose)polymerase 1 inhibition protects against age-dependent endothelial dysfunction.Clin Exp Pharmacol Physiol. 2015 Dec;42(12):1266-74. doi: 10.1111/1440-1681.12484. Clin Exp Pharmacol Physiol. 2015. PMID: 26331430
-
Neuronal trauma model: in search of Thanatos.Int J Dev Neurosci. 2004 Nov;22(7):485-96. doi: 10.1016/j.ijdevneu.2004.07.015. Int J Dev Neurosci. 2004. PMID: 15465278 Review.
Cited by
-
Inhibition of mitochondrial oxidative metabolism attenuates EMCV replication and protects β-cells from virally mediated lysis.J Biol Chem. 2020 Dec 4;295(49):16655-16664. doi: 10.1074/jbc.RA120.014851. Epub 2020 Sep 24. J Biol Chem. 2020. PMID: 32972972 Free PMC article.
-
Peroxiredoxin 1 plays a primary role in protecting pancreatic β-cells from hydrogen peroxide and peroxynitrite.Am J Physiol Regul Integr Comp Physiol. 2020 May 1;318(5):R1004-R1013. doi: 10.1152/ajpregu.00011.2020. Epub 2020 Apr 15. Am J Physiol Regul Integr Comp Physiol. 2020. PMID: 32292063 Free PMC article.
-
Vitamin E Attenuates Red-Light-Mediated Vasodilation: The Benefits of a Mild Oxidative Stress.Antioxidants (Basel). 2024 May 29;13(6):668. doi: 10.3390/antiox13060668. Antioxidants (Basel). 2024. PMID: 38929107 Free PMC article.
-
Inhibition of an NAD⁺ salvage pathway provides efficient and selective toxicity to human pluripotent stem cells.Stem Cells Transl Med. 2015 May;4(5):483-93. doi: 10.5966/sctm.2014-0163. Epub 2015 Apr 1. Stem Cells Transl Med. 2015. PMID: 25834119 Free PMC article.
-
Regulation of ATR-dependent DNA damage response by nitric oxide.J Biol Chem. 2021 Jan-Jun;296:100388. doi: 10.1016/j.jbc.2021.100388. Epub 2021 Feb 7. J Biol Chem. 2021. PMID: 33567339 Free PMC article.
References
-
- Li JM, Shah AM. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1014–R1030. - PubMed
-
- Waldman SA, Murad F. Biochemical mechanisms underlying vascular smooth muscle relaxation: the guanylate cyclase-cyclic GMP system. J Cardiovasc Pharmacol. 1988;12(Suppl 5):S115–S118. - PubMed
-
- Paravicini TM, Touyz RM. NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities. Diabetes Care. 2008;31(Suppl 2):S170–S180. - PubMed
-
- Cadenas E. Antioxidant and prooxidant functions of DT-diaphorase in quinone metabolism. Biochem Pharmacol. 1995;49:127–140. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

