Dose selection trial of metronomic oral vinorelbine monotherapy in patients with metastatic cancer: a hellenic cooperative oncology group clinical translational study
- PMID: 23718900
- PMCID: PMC3674943
- DOI: 10.1186/1471-2407-13-263
Dose selection trial of metronomic oral vinorelbine monotherapy in patients with metastatic cancer: a hellenic cooperative oncology group clinical translational study
Abstract
Background: Metronomic chemotherapy is considered an anti-angiogenic therapy that involves chronic administration of low-dose chemotherapy at regular short intervals. We investigated the optimal metronomic dose of oral vinorelbine when given as monotherapy in patients with metastatic cancer.
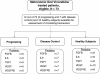
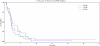
Methods: Patients with recurrent metastatic breast (BC), prostate (PC) or non-small cell lung cancer (NSCLC) and adequate organ functions were randomly assigned to 30, 40 or 50 mg vinorelbine, taken orally three times a week. Treatment continued until disease progression, unacceptable toxicity, withdrawal of consent or maximum 24 months. Primary endpoint was time-to-treatment failure (TTF) and secondary were progression-free survival (PFS), toxicity, changes in blood concentrations of angiogenesis-associated biomarkers and pharmacokinetics.
Results: Seventy-three patients were enrolled. Four-month TTF rate did not differ between the three arms: 25.9% (11.1%-46.2% 95% Confidence Interval), 33.3% (15.6%-55.3%) and 18.2% (5.2%-40.3%) for the 30 mg, 40 mg and 50 mg arms (p-value = 0.56). Objective response was seen in 2 patients with NSCLC (treated at 30 and 50 mg respectively), one with BC (at 40 m g) and one with PC (at 50 mg) and lasted from 4 to 100 weeks, with maximum response duration achieved at 50 mg. Adverse events were mild and negligible and did not differ between the three arms. Blood levels of vinorelbine reached steady state from the second week of treatment and mean values for the 30, 40 and 50 mg were respectively 1.8 ng/ml (SD 1.10), 2.2 ng/ml (SD 1.87) and 2.6 ng/ml (SD 0.69). Low pre-treatment blood concentrations of FGF2 and IL8 predicted favorable response to therapy (p values 0.02 and 0.006, respectively), while high levels of TEK gene transcript predicted treatment resistance.
Conclusions: Considering the antitumor activity and response duration, the negligible toxicity of the highest dose investigated and the lack of drug accumulation over time, we suggest that 50 mg given three times a week is the optimal dose for metronomic oral vinorelbine. Further investigation of metronomic oral vinorelbine (MOVIN) at this dose is warranted in combination with conventional chemotherapy regimens and targeted therapies.
Trial registration: Clinicaltrials.gov NCT00278070.
Figures



Similar articles
-
[Oral vinorelbine: pharmacology and treatment outcome in non-small cell bronchial carcinoma and breast carcinoma].Onkologie. 2006 Mar;29 Suppl 1:1-28. doi: 10.1159/000091889. Epub 2006 Mar 3. Onkologie. 2006. PMID: 16534241 Review. German.
-
Safety of First-line Chemotherapy with Metronomic Single-agent Oral Vinorelbine in Elderly Patients with NSCLC.Anticancer Res. 2017 Jun;37(6):3189-3194. doi: 10.21873/anticanres.11679. Anticancer Res. 2017. PMID: 28551663 Clinical Trial.
-
Metronomic oral vinorelbine in advanced breast cancer and non-small-cell lung cancer: current status and future development.Future Oncol. 2016 Feb;12(3):373-87. doi: 10.2217/fon.15.306. Epub 2015 Nov 19. Future Oncol. 2016. PMID: 26584409 Review.
-
Metronomic oral vinorelbine as first-line treatment in elderly patients with advanced non-small cell lung cancer: results of a phase II trial (MOVE trial).BMC Cancer. 2015 May 6;15:359. doi: 10.1186/s12885-015-1354-2. BMC Cancer. 2015. PMID: 25943747 Free PMC article. Clinical Trial.
-
Efficacy and safety of metronomic oral vinorelbine and its combination therapy as second- and later-line regimens for advanced non-small-cell lung cancer: a retrospective analysis.Clin Transl Oncol. 2024 Dec;26(12):3202-3210. doi: 10.1007/s12094-024-03543-z. Epub 2024 Jun 9. Clin Transl Oncol. 2024. PMID: 38851648
Cited by
-
Clinical, pharmacodynamic and pharmacokinetic results of a prospective phase II study on oral metronomic vinorelbine and dexamethasone in castration-resistant prostate cancer patients.Invest New Drugs. 2016 Dec;34(6):760-770. doi: 10.1007/s10637-016-0385-0. Epub 2016 Aug 24. Invest New Drugs. 2016. PMID: 27557783 Clinical Trial.
-
Metronomic combination of Vinorelbine and 5Fluorouracil is able to inhibit triple-negative breast cancer cells. Results from the proof-of-concept VICTOR-0 study.Oncotarget. 2018 Jun 8;9(44):27448-27459. doi: 10.18632/oncotarget.25422. eCollection 2018 Jun 8. Oncotarget. 2018. PMID: 29937997 Free PMC article.
-
Chemotherapy in frail elderly patients with hormone-refractory prostate cancer: A "real world" experience.Prostate Int. 2016 Mar;4(1):15-9. doi: 10.1016/j.prnil.2015.12.003. Epub 2016 Jan 21. Prostate Int. 2016. PMID: 27014659 Free PMC article.
-
Implementing the EffTox dose-finding design in the Matchpoint trial.BMC Med Res Methodol. 2017 Jul 20;17(1):112. doi: 10.1186/s12874-017-0381-x. BMC Med Res Methodol. 2017. PMID: 28728594 Free PMC article. Clinical Trial.
-
A Phase I Comparative Pharmacokinetic and Safety Study of Two Intravenous Formulations of Vinorelbine in Patients With Advanced Non-Small Cell Lung Cancer.Front Pharmacol. 2019 Jul 11;10:774. doi: 10.3389/fphar.2019.00774. eCollection 2019. Front Pharmacol. 2019. PMID: 31354489 Free PMC article.
References
-
- Kosmidis PA, Fountzilas G, Eleftheraki AG, Kalofonos HP, Pentheroudakis G, Skarlos D, Dimopoulos MA, Bafaloukos D, Pectasides D, Samantas E. et al.Paclitaxel and gemcitabine versus paclitaxel and vinorelbine in patients with advanced non-small-cell lung cancer. A phase III study of the hellenic cooperative oncology group (HeCOG) Ann Oncol. 2011;22(4):827–834. doi: 10.1093/annonc/mdq445. - DOI - PubMed
-
- Richards L. Targeted therapies: disappointing outcomes for anti-VEGF therapy. Nat Rev Clin Oncol. 2011;8(4):194. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

