Optical coherence tomography in retinopathy of prematurity: looking beyond the vessels
- PMID: 23719310
- PMCID: PMC3947541
- DOI: 10.1016/j.clp.2013.02.007
Optical coherence tomography in retinopathy of prematurity: looking beyond the vessels
Abstract
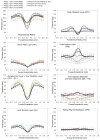
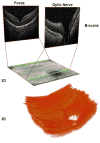
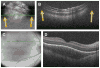
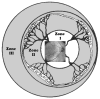
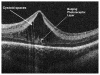
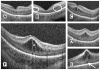
Retinopathy of Prematurity (ROP) is a leading cause of childhood blindness in the United States. Optical Coherence Tomography (OCT) is a relatively new imaging technology capable of imaging ocular structures in cross section at high resolution. We present an age-customized approach to perform Spectral Domain OCT in neonates and infants, and from SDOCT, the in-vivo development of the human fovea during the premature period up through term birth along with retinal changes unique to premature infants with ROP. Finally, we explore how this novel information may affect our understanding of ROP and the possible implications in vision and retinal development.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures















References
-
- Wojtkowski M, Leitgeb R, Kowalczyk A, et al. In vivo human retinal imaging by Fourier domain optical coherence tomography. J Biomed Opt. 2002;7(3):457–63. - PubMed
-
- Yaqoob Z, Wu J, Yang C. Spectral domain optical coherence tomography: a better OCT imaging strategy. Biotechniques. 2005;39(Suppl 6):S6–13. - PubMed
-
- Liu B, Brezinski ME. Theoretical and practical considerations on detection performance of time domain, Fourier domain, and swept source optical coherence tomography. J Biomed Opt. 2012;12(4):044007. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

