Pneumonia and pneumonia related mortality in patients with COPD treated with fixed combinations of inhaled corticosteroid and long acting β2 agonist: observational matched cohort study (PATHOS)
- PMID: 23719639
- PMCID: PMC3666306
- DOI: 10.1136/bmj.f3306
Pneumonia and pneumonia related mortality in patients with COPD treated with fixed combinations of inhaled corticosteroid and long acting β2 agonist: observational matched cohort study (PATHOS)
Erratum in
- BMJ. 2013;346:f4375
Abstract
Objective: To investigate the occurrence of pneumonia and pneumonia related events in patients with chronic obstructive pulmonary disease (COPD) treated with two different fixed combinations of inhaled corticosteroid/long acting β2 agonist.
Design: Observational retrospective pairwise cohort study matched (1:1) for propensity score.
Setting: Primary care medical records data linked to Swedish hospital, drug, and cause of death registry data for years 1999-2009.
Participants: Patients with COPD diagnosed by a physician and prescriptions of either budesonide/formoterol or fluticasone/salmeterol.
Main outcome measures: Yearly pneumonia event rates, admission to hospital related to pneumonia, and mortality.
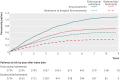
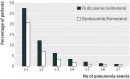
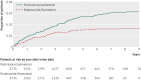
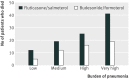
Results: 9893 patients were eligible for matching (2738 in the fluticasone/salmeterol group; 7155 in the budesonide/formoterol group), yielding two matched cohorts of 2734 patients each. In these patients, 2115 (39%) had at least one recorded episode of pneumonia during the study period, with 2746 episodes recorded during 19,170 patient years of follow up. Compared with budesonide/formoterol, rate of pneumonia and admission to hospital were higher in patients treated with fluticasone/salmeterol: rate ratio 1.73 (95% confidence interval 1.57 to 1.90; P<0.001) and 1.74 (1.56 to 1.94; P<0.001), respectively. The pneumonia event rate per 100 patient years for fluticasone/salmeterol versus budesonide/formoterol was 11.0 (10.4 to 11.8) versus 6.4 (6.0 to 6.9) and the rate of admission to hospital was 7.4 (6.9 to 8.0) versus 4.3 (3.9 to 4.6). The mean duration of admissions related to pneumonia was similar for both groups, but mortality related to pneumonia was higher in the fluticasone/salmeterol group (97 deaths) than in the budesonide/formoterol group (52 deaths) (hazard ratio 1.76, 1.22 to 2.53; P=0.003). All cause mortality did not differ between the treatments (1.08, 0.93 to 1.14; P=0.59).
Conclusions: There is an intra-class difference between fixed combinations of inhaled corticosteroid/long acting β2 agonist with regard to the risk of pneumonia and pneumonia related events in the treatment of patients with COPD.
Trial registration: Clinical Trials.gov NCT01146392.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures

References
-
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. Updated 2011. www.goldcopd.org/. - PubMed
-
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 2006;367:1747-57. - PubMed
-
- Calverley PM, Stockley RA, Seemungal TA, Hagan G, Willits LR, Riley JH, et al. Reported pneumonia in patients with COPD: findings from the INSPIRE study. Chest 2011;139:505-12. - PubMed
-
- Holguin F, Folch E, Redd SC, Mannino DM. Comorbidity and mortality in COPD-related hospitalizations in the United States, 1979 to 2001. Chest 2005;128:2005-11. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
