Astragaloside IV attenuates glycated albumin-induced epithelial-to-mesenchymal transition by inhibiting oxidative stress in renal proximal tubular cells
- PMID: 23719694
- PMCID: PMC3857426
- DOI: 10.1007/s12192-013-0438-7
Astragaloside IV attenuates glycated albumin-induced epithelial-to-mesenchymal transition by inhibiting oxidative stress in renal proximal tubular cells
Abstract
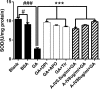
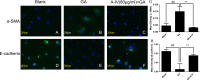
In diabetic kidney disease (DKD), epithelial-to-mesenchymal transition (EMT) is a classic pathological process in tubular damage. Oxidative stress is considered to play an important role in DKD. Astragaloside IV (A-IV), one of the main active ingredients of Astragalus membranaceus, exhibits a wide range of biological activities. However, the effect of A-IV on regulating EMT in tubular cells is unclear. This study aims to determine whether A-IV could attenuate glycated albumin (GA)-induced EMT in the NRK-52E cell line by inhibiting oxidative stress. GA and A-IV-induced cytotoxicity were assayed by CCK-8. The intercellular reactive oxygen species (ROS) level was detected by H2DCFDA. The activity of NADPH oxidase was assayed by adding exogenous NADPH oxidase, and the superoxide dismutase (SOD) units were observed by NBT. We used a microscope to examine the morphology of the NRK-52E cell line. We conducted a wound healing assay to measure cell mobility. To determine mRNA and protein expressions of α-SMA and E-cadherin, we used real-time polymerase chain reaction (real-time PCR), immunofluorescence, and western blot analysis. A-IV significantly attenuated GA-induced amplification of ROS, lowered the increased level of NADPH oxidase activity, and elevated the decreased level of SOD units. The GA-induced NRK-52E cell line showed increased expression of α-SMA and decreased expression of E-cadherin in mRNA and protein levels, whereas A-IV alleviated the expression of α-SMA and increased the expression of E-cadherin. Our data demonstrate that GA could induce NRK-52E cell line EMT through oxidative stress. This effect could be attenuated by A-IV via regulation of the impaired redox balance.
Figures





References
-
- Burns WC, Thomas MC. The molecular mediators of type 2 epithelial to mesenchymal transition (EMT) and their role in renal pathophysiology. Expert Rev Mol Med. 2010;27:12–17. - PubMed
-
- Chang YX, Sun YG, Li J, Zhang QH, Guo XR, Zhang BL, et al. The experimental study of Astragalus membranaceus on meridian tropsim: the distribution study of astragaloside IV in rat tissues. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;12(911):71–5. doi: 10.1016/j.jchromb.2012.10.024. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

