Conflicts between local and global spatial frameworks dissociate neural representations of the lateral and medial entorhinal cortex
- PMID: 23719794
- PMCID: PMC3747988
- DOI: 10.1523/JNEUROSCI.0946-13.2013
Conflicts between local and global spatial frameworks dissociate neural representations of the lateral and medial entorhinal cortex
Erratum in
- J Neurosci. 2013 Aug 7;33(32):13249
Abstract
Manipulation of spatial reference frames is a common experimental tool to investigate the nature of hippocampal information coding and to investigate high-order processes, such as cognitive coordination. However, it is unknown how the hippocampus afferents represent the local and global reference frames of an environment. To address these issues, single units were recorded in freely moving rats with multi-tetrode arrays targeting the superficial layers of the lateral entorhinal cortex (LEC) and medial entorhinal cortex (MEC), the two primary cortical inputs to the hippocampus. Rats ran clockwise laps around a circular track partitioned into quadrants covered by different textures (the local reference frame). The track was centered in a circular environment with distinct landmarks on the walls (the global reference frame). Here we demonstrate a novel dissociation between MEC and LEC in that the global frame controlled the MEC representation and the local frame controlled the LEC representation when the reference frames were rotated in equal, but opposite, directions. Consideration of the functional anatomy of the hippocampal circuit and popular models of attractor dynamics in CA3 suggests a mechanistic explanation of previous data showing a dissociation between the CA3 and CA1 regions in their responses to this local-global conflict. Furthermore, these results are consistent with a model of the LEC providing the hippocampus with the external sensory content of an experience and the MEC providing the spatial context, which combine to form conjunctive codes in the hippocampus that form the basis of episodic memory.
Figures
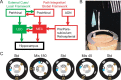


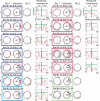
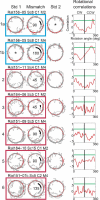
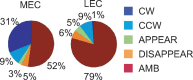
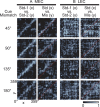
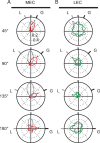
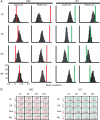
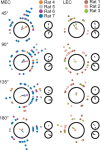
Comment in
-
Location memory: separate cortical coding for distal and local cues.Curr Biol. 2013 Aug 19;23(16):R685-7. doi: 10.1016/j.cub.2013.07.018. Curr Biol. 2013. PMID: 23968923
Similar articles
-
Distal CA1 Maintains a More Coherent Spatial Representation than Proximal CA1 When Local and Global Cues Conflict.J Neurosci. 2021 Nov 24;41(47):9767-9781. doi: 10.1523/JNEUROSCI.2938-20.2021. Epub 2021 Oct 20. J Neurosci. 2021. PMID: 34670850 Free PMC article.
-
Complementary Functional Organization of Neuronal Activity Patterns in the Perirhinal, Lateral Entorhinal, and Medial Entorhinal Cortices.J Neurosci. 2016 Mar 30;36(13):3660-75. doi: 10.1523/JNEUROSCI.4368-15.2016. J Neurosci. 2016. PMID: 27030753 Free PMC article.
-
Differential roles of medial/lateral entorhinal cortex in spatial/object memory and contribution to hippocampal functional neuronal organization.Neurobiol Learn Mem. 2025 Jan;217:108015. doi: 10.1016/j.nlm.2024.108015. Epub 2024 Dec 15. Neurobiol Learn Mem. 2025. PMID: 39689754
-
Functional correlates of the lateral and medial entorhinal cortex: objects, path integration and local-global reference frames.Philos Trans R Soc Lond B Biol Sci. 2013 Dec 23;369(1635):20130369. doi: 10.1098/rstb.2013.0369. Print 2014 Feb 5. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 24366146 Free PMC article. Review.
-
Tracking the flow of hippocampal computation: Pattern separation, pattern completion, and attractor dynamics.Neurobiol Learn Mem. 2016 Mar;129:38-49. doi: 10.1016/j.nlm.2015.10.008. Epub 2015 Oct 26. Neurobiol Learn Mem. 2016. PMID: 26514299 Free PMC article. Review.
Cited by
-
Hippocampus Maintains a Coherent Map Under Reward Feature-Landmark Cue Conflict.Front Neural Circuits. 2022 Apr 26;16:878046. doi: 10.3389/fncir.2022.878046. eCollection 2022. Front Neural Circuits. 2022. PMID: 35558552 Free PMC article.
-
Assessing mild cognitive impairment using object-location memory in immersive virtual environments.Hippocampus. 2022 Sep;32(9):660-678. doi: 10.1002/hipo.23458. Epub 2022 Aug 2. Hippocampus. 2022. PMID: 35916343 Free PMC article.
-
Origin and role of path integration in the cognitive representations of the hippocampus: computational insights into open questions.J Exp Biol. 2019 Feb 6;222(Pt Suppl 1):jeb188912. doi: 10.1242/jeb.188912. J Exp Biol. 2019. PMID: 30728236 Free PMC article. Review.
-
The medial prefrontal cortex - hippocampus circuit that integrates information of object, place and time to construct episodic memory in rodents: Behavioral, anatomical and neurochemical properties.Neurosci Biobehav Rev. 2020 Jun;113:373-407. doi: 10.1016/j.neubiorev.2020.04.007. Epub 2020 Apr 13. Neurosci Biobehav Rev. 2020. PMID: 32298711 Free PMC article. Review.
-
Medial Entorhinal Cortex Selectively Supports Temporal Coding by Hippocampal Neurons.Neuron. 2017 May 3;94(3):677-688.e6. doi: 10.1016/j.neuron.2017.04.003. Epub 2017 Apr 20. Neuron. 2017. PMID: 28434800 Free PMC article.
References
-
- Brown JE, Skaggs WE. Concordant and discordant coding of spatial location in populations of hippocampal CA1 pyramidal cells. J Neurophysiol. 2002;88:1605–1613. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
