Tau accumulation activates the unfolded protein response by impairing endoplasmic reticulum-associated degradation
- PMID: 23719816
- PMCID: PMC3733249
- DOI: 10.1523/JNEUROSCI.5397-12.2013
Tau accumulation activates the unfolded protein response by impairing endoplasmic reticulum-associated degradation
Abstract
In Alzheimer's disease (AD), the mechanisms of neuronal loss remain largely unknown. Although tau pathology is closely correlated with neuronal loss, how its accumulation may lead to activation of neurotoxic pathways is unclear. Here we show that tau increased the levels of ubiquitinated proteins in the brain and triggered activation of the unfolded protein response (UPR). This suggested that tau interferes with protein quality control in the endoplasmic reticulum (ER). Consistent with this, ubiquitin was found to associate with the ER in human AD brains and tau transgenic (rTg4510) mouse brains, but this was not always colocalized with tau. The increased levels of ubiquitinated protein were accompanied by increased levels of phosphorylated protein kinase R-like ER kinase (pPERK), a marker that indicates UPR activation. Depleting soluble tau levels in cells and brain could reverse UPR activation. Tau accumulation facilitated its deleterious interaction with ER membrane and associated proteins that are essential for ER-associated degradation (ERAD), including valosin-containing protein (VCP) and Hrd1. Based on this, the effects of tau accumulation on ERAD efficiency were evaluated using the CD3δ reporter, an ERAD substrate. Indeed, CD3δ accumulated in both in vitro and in vivo models of tau overexpression and AD brains. These data suggest that soluble tau impairs ERAD and the result is activation of the UPR. The reversibility of this process, however, suggests that tau-based therapeutics could significantly delay this type of cell death and therefore disease progression.
Figures
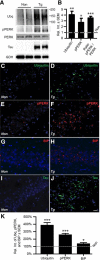
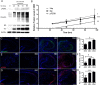
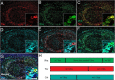




Comment in
-
Tau pathology as a cause and consequence of the UPR.J Neurosci. 2013 Sep 4;33(36):14285-7. doi: 10.1523/JNEUROSCI.2961-13.2013. J Neurosci. 2013. PMID: 24005281 Free PMC article. No abstract available.
References
-
- Abisambra JF, Blair LJ, Hill SE, Jones JR, Kraft C, Rogers J, Koren J, 3rd, Jinwal UK, Lawson L, Johnson AG, Wilcock D, O'Leary JC, Jansen-West K, Muschol M, Golde TE, Weeber EJ, Banko J, Dickey CA. Phosphorylation dynamics regulate Hsp27-mediated rescue of neuronal plasticity deficits in tau transgenic mice. J Neurosci. 2010b;30:15374–15382. doi: 10.1523/JNEUROSCI.3155-10.2010. - DOI - PMC - PubMed
-
- Bancher C, Brunner C, Lassmann H, Budka H, Jellinger K, Seitelberger F, Grundke-Iqbal I, Iqbal K, Wisniewski HM. Tau and ubiquitin immunoreactivity at different stages of formation of Alzheimer neurofibrillary tangles. Prog Clin Biol Res. 1989;317:837–848. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
