Lack of conventional oxygen-linked proton and anion binding sites does not impair allosteric regulation of oxygen binding in dwarf caiman hemoglobin
- PMID: 23720132
- PMCID: PMC3743003
- DOI: 10.1152/ajpregu.00014.2013
Lack of conventional oxygen-linked proton and anion binding sites does not impair allosteric regulation of oxygen binding in dwarf caiman hemoglobin
Abstract
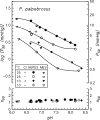
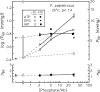
In contrast to other vertebrate hemoglobins (Hbs) whose high intrinsic O2 affinities are reduced by red cell allosteric effectors (mainly protons, CO2, organic phosphates, and chloride ions), crocodilian Hbs exhibit low sensitivity to organic phosphates and high sensitivity to bicarbonate (HCO3(-)), which is believed to augment Hb-O2 unloading during diving and postprandial alkaline tides when blood HCO3(-) levels and metabolic rates increase. Examination of α- and β-globin amino acid sequences of dwarf caiman (Paleosuchus palpebrosus) revealed a unique combination of substitutions at key effector binding sites compared with other vertebrate and crocodilian Hbs: β82Lys→Gln, β143His→Val, and β146His→Tyr. These substitutions delete positive charges and, along with other distinctive changes in residue charge and polarity, may be expected to disrupt allosteric regulation of Hb-O2 affinity. Strikingly, however, P. palpebrosus Hb shows a strong Bohr effect, and marked deoxygenation-linked binding of organic phosphates (ATP and DPG) and CO2 as carbamate (contrasting with HCO3(-) binding in other crocodilians). Unlike other Hbs, it polymerizes to large complexes in the oxygenated state. The highly unusual properties of P. palpebrosus Hb align with a high content of His residues (potential sites for oxygenation-linked proton binding) and distinctive surface Cys residues that may form intermolecular disulfide bridges upon polymerization. On the basis of its singular properties, P. palpebrosus Hb provides a unique opportunity for studies on structure-function coupling and the evolution of compensatory mechanisms for maintaining tissue O2 delivery in Hbs that lack conventional effector-binding residues.
Keywords: Bohr effect; allosteric interaction; carbon dioxide; crocodilians; oxygen-binding.
Figures






References
-
- Ackers GK. Molecular exclusion and restricted diffusion processes in molecular-sieve chromatography. Biochemistry 3: 723–730, 1964 - PubMed
-
- Amiconi G, Bertollini A, Bellelli A, Coletta M, Condò SG, Brunori M. Evidence for two oxygen-linked binding sites for polyanions in dromedary hemoglobin. Eur J Biochem 150: 387–393, 1985 - PubMed
-
- Antonini E, Wyman J, Brunori M, Fronticelli C, Bucci E, Rossi-Fanelli A. Studies on the relations between molecular and functional properties of hemoglobin. V. The influence of temperature on the Bohr effect in human and in horse hemoglobin. J Biol Chem 240: 1096–1103, 1965 - PubMed
-
- Atha DH, Ackers GK. Calorimetric determination of the heat of oxygenation of human hemoglobin as a function of pH and the extent of reaction. Biochemistry 13: 2376–2382, 1974 - PubMed
-
- Bårdgard A, Fago A, Malte H, Weber RE. Oxygen binding and aggregation of hemoglobin from the common European frog, Rana temporaria. Comp Biochem Physiol B Biochem Mol Biol 117: 225–231, 1997 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

