Pontine mechanisms of respiratory control
- PMID: 23720253
- PMCID: PMC4422496
- DOI: 10.1002/cphy.c100015
Pontine mechanisms of respiratory control
Abstract
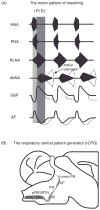
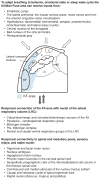
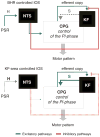
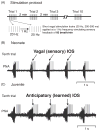
Pontine respiratory nuclei provide synaptic input to medullary rhythmogenic circuits to shape and adapt the breathing pattern. An understanding of this statement depends on appreciating breathing as a behavior, rather than a stereotypic rhythm. In this review, we focus on the pontine-mediated inspiratory off-switch (IOS) associated with postinspiratory glottal constriction. Further, IOS is examined in the context of pontine regulation of glottal resistance in response to multimodal sensory inputs and higher commands, which in turn rules timing, duration, and patterning of respiratory airflow. In addition, network plasticity in respiratory control emerges during the development of the pons. Synaptic plasticity is required for dynamic and efficient modulation of the expiratory breathing pattern to cope with rapid changes from eupneic to adaptive breathing linked to exploratory (foraging and sniffing) and expulsive (vocalizing, coughing, sneezing, and retching) behaviors, as well as conveyance of basic emotions. The speed and complexity of changes in the breathing pattern of behaving animals implies that "learning to breathe" is necessary to adjust to changing internal and external states to maintain homeostasis and survival.
© 2012 American Physiological Society
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

