The cellular building blocks of breathing
- PMID: 23720262
- PMCID: PMC3684023
- DOI: 10.1002/cphy.c110033
The cellular building blocks of breathing
Abstract
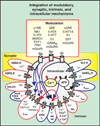
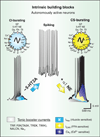
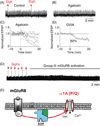
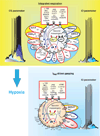
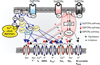
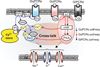
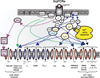
Respiratory brainstem neurons fulfill critical roles in controlling breathing: they generate the activity patterns for breathing and contribute to various sensory responses including changes in O2 and CO2. These complex sensorimotor tasks depend on the dynamic interplay between numerous cellular building blocks that consist of voltage-, calcium-, and ATP-dependent ionic conductances, various ionotropic and metabotropic synaptic mechanisms, as well as neuromodulators acting on G-protein coupled receptors and second messenger systems. As described in this review, the sensorimotor responses of the respiratory network emerge through the state-dependent integration of all these building blocks. There is no known respiratory function that involves only a small number of intrinsic, synaptic, or modulatory properties. Because of the complex integration of numerous intrinsic, synaptic, and modulatory mechanisms, the respiratory network is capable of continuously adapting to changes in the external and internal environment, which makes breathing one of the most integrated behaviors. Not surprisingly, inspiration is critical not only in the control of ventilation, but also in the context of "inspiring behaviors" such as arousal of the mind and even creativity. Far-reaching implications apply also to the underlying network mechanisms, as lessons learned from the respiratory network apply to network functions in general.
© 2012 American Physiological Society
Figures








References
-
- Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, MacDonald JF, Tymianski M. Akey role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–877. - PubMed
-
- Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement JP, th, Boyd AE, 3rd, Gonzalez G, Herrera-Sosa H, Nguy K, Bryan J, Nelson DA. Cloning of the beta cell high-affinity sulfonylurea receptor: A regulator of insulin secretion. Science. 1995;268:423–426. - PubMed
-
- Ahmmed GU, Mehta D, Vogel S, Holinstat M, Paria BC, Tiruppathi C, Malik AB. Protein kinase Calpha phosphorylates the TRPC1 channel and regulates store-operated Ca2+ entry in endothelial cells. J Biol Chem. 2004;279:20941–20949. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

