Nonclassical renin-angiotensin system and renal function
- PMID: 23720263
- PMCID: PMC4186703
- DOI: 10.1002/cphy.c120002
Nonclassical renin-angiotensin system and renal function
Abstract
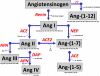
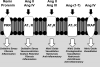
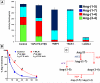
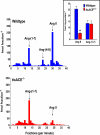
The renin-angiotensin system (RAS) constitutes one of the most important hormonal systems in the physiological regulation of blood pressure through renal and nonrenal mechanisms. Indeed, dysregulation of the RAS is considered a major factor in the development of cardiovascular pathologies, including kidney injury, and blockade of this system by the inhibition of angiotensin converting enzyme (ACE) or blockade of the angiotensin type 1 receptor (AT1R) by selective antagonists constitutes an effective therapeutic regimen. It is now apparent with the identification of multiple components of the RAS within the kidney and other tissues that the system is actually composed of different angiotensin peptides with diverse biological actions mediated by distinct receptor subtypes. The classic RAS can be defined as the ACE-Ang II-AT1R axis that promotes vasoconstriction, water intake, sodium retention, and other mechanisms to maintain blood pressure, as well as increase oxidative stress, fibrosis, cellular growth, and inflammation in pathological conditions. In contrast, the nonclassical RAS composed primarily of the AngII/Ang III-AT2R pathway and the ACE2-Ang-(1-7)-AT7R axis generally opposes the actions of a stimulated Ang II-AT1R axis through an increase in nitric oxide and prostaglandins and mediates vasodilation, natriuresis, diuresis, and reduced oxidative stress. Moreover, increasing evidence suggests that these non-classical RAS components contribute to the therapeutic blockade of the classical system to reduce blood pressure and attenuate various indices of renal injury, as well as contribute to normal renal function.
© 2012 American Physiological Society
Figures
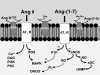
References
-
- Abadir PM, Carey RM, Siragy HM. Angiotensin AT2 receptors directly stimulate renal nitric oxide in bradykinin B2-receptor null mice. Hypertension. 2003;42:600–604. - PubMed
-
- Ahmad A, Ward PE. Role of aminopeptidase activity in the regulation of the pressor activity of circulating angiotensins. J Pharmacol Exp Ther. 1990;252:643–650. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

