Renal ammonia metabolism and transport
- PMID: 23720285
- PMCID: PMC4319187
- DOI: 10.1002/cphy.c120010
Renal ammonia metabolism and transport
Abstract
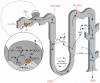
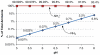
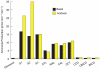
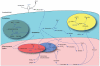
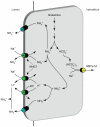
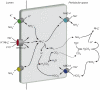
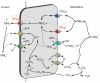
Renal ammonia metabolism and transport mediates a central role in acid-base homeostasis. In contrast to most renal solutes, the majority of renal ammonia excretion derives from intrarenal production, not from glomerular filtration. Renal ammoniagenesis predominantly results from glutamine metabolism, which produces 2 NH4(+) and 2 HCO3(-) for each glutamine metabolized. The proximal tubule is the primary site for ammoniagenesis, but there is evidence for ammoniagenesis by most renal epithelial cells. Ammonia produced in the kidney is either excreted into the urine or returned to the systemic circulation through the renal veins. Ammonia excreted in the urine promotes acid excretion; ammonia returned to the systemic circulation is metabolized in the liver in a HCO3(-)-consuming process, resulting in no net benefit to acid-base homeostasis. Highly regulated ammonia transport by renal epithelial cells determines the proportion of ammonia excreted in the urine versus returned to the systemic circulation. The traditional paradigm of ammonia transport involving passive NH3 diffusion, protonation in the lumen and NH4(+) trapping due to an inability to cross plasma membranes is being replaced by the recognition of limited plasma membrane NH3 permeability in combination with the presence of specific NH3-transporting and NH4(+)-transporting proteins in specific renal epithelial cells. Ammonia production and transport are regulated by a variety of factors, including extracellular pH and K(+), and by several hormones, such as mineralocorticoids, glucocorticoids and angiotensin II. This coordinated process of regulated ammonia production and transport is critical for the effective maintenance of acid-base homeostasis.
Figures


References
-
- Adam WR, Koretsky AP, Weiner MW. 31P-NMR in vivo measurement of renal intracellular pH: Effects of acidosis and K+ depletion in rats. Am J Physiol. 1986;251:F904–F910. - PubMed
-
- Adam WR, Simpson DP. Renal mitochondrial glutamine metabolism and dietary potassium and protein content. Kidney Int. 1975;7:325–330. - PubMed
-
- Adrogue HJ. Glucose homeostasis and the kidney. Kidney Int. 1992;42:1266–1282. - PubMed
-
- Alleyne GA, Barnswell J, McFarlane-Anderson N, Alexander JE. Renal ammoniagenic factor in the plasma of rats with acute metabolic acidosis. Am J Physiol. 1981;241:F112–F116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

