Hepatocyte polarity
- PMID: 23720287
- PMCID: PMC3697931
- DOI: 10.1002/cphy.c120009
Hepatocyte polarity
Abstract
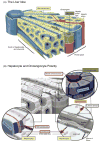
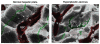
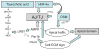
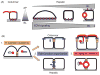
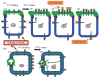
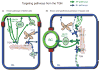
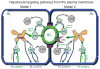
Hepatocytes, like other epithelia, are situated at the interface between the organism's exterior and the underlying internal milieu and organize the vectorial exchange of macromolecules between these two spaces. To mediate this function, epithelial cells, including hepatocytes, are polarized with distinct luminal domains that are separated by tight junctions from lateral domains engaged in cell-cell adhesion and from basal domains that interact with the underlying extracellular matrix. Despite these universal principles, hepatocytes distinguish themselves from other nonstriated epithelia by their multipolar organization. Each hepatocyte participates in multiple, narrow lumina, the bile canaliculi, and has multiple basal surfaces that face the endothelial lining. Hepatocytes also differ in the mechanism of luminal protein trafficking from other epithelia studied. They lack polarized protein secretion to the luminal domain and target single-spanning and glycosylphosphatidylinositol-anchored bile canalicular membrane proteins via transcytosis from the basolateral domain. We compare this unique hepatic polarity phenotype with that of the more common columnar epithelial organization and review our current knowledge of the signaling mechanisms and the organization of polarized protein trafficking that govern the establishment and maintenance of hepatic polarity. The serine/threonine kinase LKB1, which is activated by the bile acid taurocholate and, in turn, activates adenosine monophosphate kinase-related kinases including AMPK1/2 and Par1 paralogues has emerged as a key determinant of hepatic polarity. We propose that the absence of a hepatocyte basal lamina and differences in cell-cell adhesion signaling that determine the positioning of tight junctions are two crucial determinants for the distinct hepatic and columnar polarity phenotypes.
Figures




References
-
- Ala A, Walker AP, Ashkan K, Dooley JS, Schilsky ML. Wilson’s disease. Lancet. 2007;369:397–408. - PubMed
-
- Alessi DR, Sakamoto K, Bayascas JR. LKB1-dependent signaling pathways. Annu Rev Biochem. 2006;75:137–163. - PubMed
-
- Almeida CG, Yamada A, Tenza D, Louvard D, Raposo G, Coudrier E. Myosin 1b promotes the formation of post-Golgi carriers by regulating actin assembly and membrane remodelling at the trans-Golgi network. Nat Cell Biol. 2011;13:779–789. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

