The influence of exercise on cognitive abilities
- PMID: 23720292
- PMCID: PMC3951958
- DOI: 10.1002/cphy.c110063
The influence of exercise on cognitive abilities
Abstract
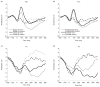
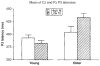
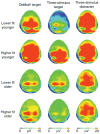
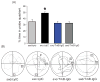
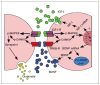
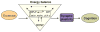
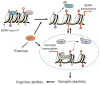
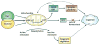
Scientific evidence based on neuroimaging approaches over the last decade has demonstrated the efficacy of physical activity improving cognitive health across the human lifespan. Aerobic fitness spares age-related loss of brain tissue during aging, and enhances functional aspects of higher order regions involved in the control of cognition. More active or higher fit individuals are capable of allocating greater attentional resources toward the environment and are able to process information more quickly. These data are suggestive that aerobic fitness enhances cognitive strategies enabling to respond effectively to an imposed challenge with a better yield in task performance. In turn, animal studies have shown that exercise has a benevolent action on health and plasticity of the nervous system. New evidence indicates that exercise exerts its effects on cognition by affecting molecular events related to the management of energy metabolism and synaptic plasticity. An important instigator in the molecular machinery stimulated by exercise is brain-derived neurotrophic factor, which acts at the interface of metabolism and plasticity. Recent studies show that exercise collaborates with other aspects of lifestyle to influence the molecular substrates of cognition. In particular, select dietary factors share similar mechanisms with exercise, and in some cases they can complement the action of exercise. Therefore, exercise and dietary management appear as a noninvasive and effective strategy to counteract neurological and cognitive disorders.
Figures






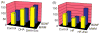

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

