Physiology of cholangiocytes
- PMID: 23720296
- PMCID: PMC3831353
- DOI: 10.1002/cphy.c120019
Physiology of cholangiocytes
Abstract
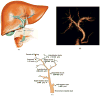
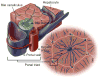
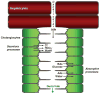
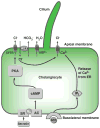
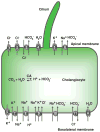
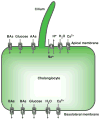
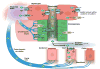
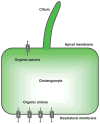
Cholangiocytes are epithelial cells that line the intra- and extrahepatic ducts of the biliary tree. The main physiologic function of cholangiocytes is modification of hepatocyte-derived bile, an intricate process regulated by hormones, peptides, nucleotides, neurotransmitters, and other molecules through intracellular signaling pathways and cascades. The mechanisms and regulation of bile modification are reviewed herein.
Figures


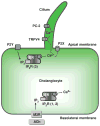
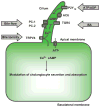
References
-
- Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: Update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. - PMC - PubMed
-
- Alper SL. Molecular physiology of SLC4 anion exchangers. Exp Physiol. 2006;91:153–161. - PubMed
-
- Alpini G, Baiocchi L, Glaser S, Ueno Y, Marzioni M, Francis H, Phinizy JL, Angelico M, Lesage G. Ursodeoxycholate and tauroursodeoxycholate inhibit cholangiocyte growth and secretion of BDL rats through activation of PKC alpha. Hepatology. 2002;35:1041–1052. - PubMed
-
- Alpini G, Franchitto A, Demorrow S, Onori P, Gaudio E, Wise C, Francis H, Venter J, Kopriva S, Mancinelli R, Carpino G, Stagnitti F, Ueno Y, Han Y, Meng F, Glaser S. Activation of alpha(1) -adrenergic receptors stimulate the growth of small mouse cholangiocytes via calcium-dependent activation of nuclear factor of activated T cells 2 and specificity protein 1. Hepatology. 2011;53:628–639. - PMC - PubMed
-
- Alpini G, Glaser S, Alvaro D, Ueno Y, Marzioni M, Francis H, Baiocchi L, Stati T, Barbaro B, Phinizy JL, Mauldin J, Lesage G. Bile acid depletion and repletion regulate cholangiocyte growth and secretion by a phosphatidylinositol 3-kinase-dependent pathway in rats. Gastroenterology. 2002;123:1226–1237. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

