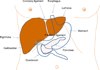
Liver immunology
- PMID: 23720323
- PMCID: PMC4201126
- DOI: 10.1002/cphy.c120011
Liver immunology
Abstract
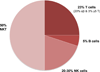
The liver is the largest organ in the body and is generally regarded by nonimmunologists as having little or no lymphoid function. However, such is far from accurate. This review highlights the importance of the liver as a lymphoid organ. Firstly, we discuss experimental data surrounding the role of liver as a lymphoid organ. The liver facilitates tolerance rather than immunoreactivity, which protects the host from antigenic overload of dietary components and drugs derived from the gut and it is instrumental to fetal immune tolerance. Loss of liver tolerance leads to autoaggressive phenomena, which if not controlled by regulatory lymphoid populations, may lead to the induction of autoimmune liver diseases. Liver-related lymphoid subpopulations also act as critical antigen-presenting cells. The study of the immunological properties of liver and delineation of the microenvironment of the intrahepatic milieu in normal and diseased livers provides a platform to understand the hierarchy of a series of detrimental events that lead to immune-mediated destruction of the liver and the rejection of liver allografts. The majority of emphasis within this review will be on the normal mononuclear cell composition of the liver. However, within this context, we will discuss selected, but not all, immune-mediated liver disease and attempt to place these data in the context of human autoimmunity.
Conflict of interest statement
Figures










References
-
- Kita H, Mackay IR, Van De Water J, Gershwin ME. The lymphoid liver: considerations on pathways to autoimmune injury. Gastroenterology. 2001;120:1485–1501. - PubMed
-
- Calne RY, Rolles K, White DJ, Thiru S, Evans DB, McMaster P, Dunn DC, Craddock GN, Henderson RG, Aziz S, Lewis P. Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreases, and 2 livers. Lancet. 1979;2:1033–1036. - PubMed
-
- Zimmermann FA, White DJ, Gokel JM, Calne RY. Orthotopic liver transplantation in rats. Prolonging of survival time of allotransplants using cyclosporin A in an acute rejection model. Chir Forum Exp Klin Forsch. 1979:339–344. - PubMed
-
- Calne RY, Sells RA, Pena JR, Davis DR, Millard PR, Herbertson BM, Binns RM, Davies DA. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;223:472–476. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

