Comparative digestive physiology
- PMID: 23720328
- PMCID: PMC4458075
- DOI: 10.1002/cphy.c110054
Comparative digestive physiology
Abstract
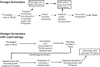
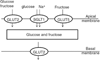
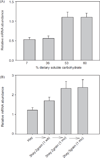
In vertebrates and invertebrates, morphological and functional features of gastrointestinal (GI) tracts generally reflect food chemistry, such as content of carbohydrates, proteins, fats, and material(s) refractory to rapid digestion (e.g., cellulose). The expression of digestive enzymes and nutrient transporters approximately matches the dietary load of their respective substrates, with relatively modest excess capacity. Mechanisms explaining differences in hydrolase activity between populations and species include gene copy number variations and single-nucleotide polymorphisms. Transcriptional and posttranscriptional adjustments mediate phenotypic changes in the expression of hydrolases and transporters in response to dietary signals. Many species respond to higher food intake by flexibly increasing digestive compartment size. Fermentative processes by symbiotic microorganisms are important for cellulose degradation but are relatively slow, so animals that rely on those processes typically possess special enlarged compartment(s) to maintain a microbiota and other GI structures that slow digesta flow. The taxon richness of the gut microbiota, usually identified by 16S rRNA gene sequencing, is typically an order of magnitude greater in vertebrates than invertebrates, and the interspecific variation in microbial composition is strongly influenced by diet. Many of the nutrient transporters are orthologous across different animal phyla, though functional details may vary (e.g., glucose and amino acid transport with K+ rather than Na+ as a counter ion). Paracellular absorption is important in many birds. Natural toxins are ubiquitous in foods and may influence key features such as digesta transit, enzymatic breakdown, microbial fermentation, and absorption.
Figures















References
-
- Abe T, Higashi M. Cellulose centered perspective on community structure. Oikos. 1991;60:127–133.
-
- Acamovic T, Brooker JD. Biochemistry of plant secondary metabolites and their effects in animals. Proc Nutr Soc. 2005;64:403–412. - PubMed
-
- Adeola O, King DE. Developmental changes in morphometry of the small intestine and jejunal sucrase activity during the first nine weeks of postnatal growth in pigs. J Anim Sci. 2006;84:112–118. - PubMed
-
- Afik D, Karasov WH. The trade-offs between digestion rate and efficiency in warblers and their ecological implications. Ecology. 1995;76:2247–2257.
-
- Allardyce BJ, Linton SM, Saborowski R. The last piece in the cellulase puzzle: The characterisation of beta-glucosidase from the herbivorous gecarcinid land crab Gecarcoidea natalis . J Exp Biol. 2010;213:2950–2957. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

