Differential regulation of Na+ transporters along nephron during ANG II-dependent hypertension: distal stimulation counteracted by proximal inhibition
- PMID: 23720346
- PMCID: PMC3891260
- DOI: 10.1152/ajprenal.00183.2013
Differential regulation of Na+ transporters along nephron during ANG II-dependent hypertension: distal stimulation counteracted by proximal inhibition
Abstract
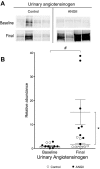
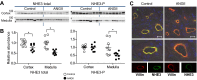
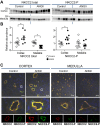
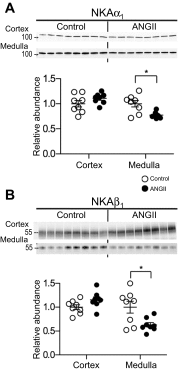
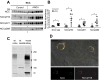
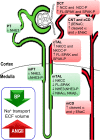
During angiotensin II (ANG II)-dependent hypertension, ANG II stimulates, while hypertension inhibits, Na(+) transporter activity to balance Na(+) output to input. This study tests the hypothesis that ANG II infusion activates Na(+) transporters in the distal nephron while inhibiting transporters along the proximal nephron. Male Sprague-Dawley rats were infused with ANG II (400 ng·kg(-1)·min(-1)) or vehicle for 2 wk. Kidneys were dissected (cortex vs. medulla) or fixed for immunohistochemistry (IHC). ANG II increased mean arterial pressure by 40 mmHg, urine Na(+) by 1.67-fold, and urine volume by 3-fold, evidence for hypertension and pressure natriuresis. Na(+) transporters' abundance and activation [assessed by phosphorylation (-P) or proteolytic cleavage] were measured by immunoblot. During ANG II infusion Na(+)/H(+) exchanger 3 (NHE3) abundance decreased in both cortex and medulla; Na-K-2Cl cotransporter 2 (NKCC2) decreased in medullary thick ascending loop of Henle (TALH) and increased, along with NKCC2-P, in cortical TALH; Na-Cl cotransporter (NCC) and NCC-P increased in the distal convoluted tubule; and epithelial Na(+) channel subunits and their cleaved forms were increased in both cortex and medulla. Like NKCC2, STE20/SPS1-related proline alanine-rich kinase (SPAK) and SPAK-P were decreased in medulla and increased in cortex. By IHC, during ANG II NHE3 remained localized to proximal tubule microvilli at lower abundance, and the differential regulation of NKCC2 and NKCC2-P in cortex versus medulla was evident. In summary, ANG II infusion increases Na(+) transporter abundance and activation from cortical TALH to medullary collecting duct while the hypertension drives a natriuresis response evident as decreased Na(+) transporter abundance and activation from proximal tubule through medullary TALH.
Keywords: ENaC; NCC; NHE3; NKCC2; SPAK; pressure natriuresis.
Figures



References
-
- Alonso-Galicia M, Maier KG, Greene AS, Cowley AW, Jr, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid in the renal and vasoconstrictor actions of angiotensin II. Am J Physiol Regul Integr Comp Physiol 283: R60–R68, 2002 - PubMed
-
- Banday AA, Lokhandwala MF. Oxidative stress causes renal angiotensin II type 1 receptor upregulation, Na+/H+ exchanger 3 overstimulation, and hypertension. Hypertension 57: 452–459, 2011 - PubMed
-
- Beutler KT, Masilamani S, Turban S, Nielsen J, Brooks HL, Ageloff S, Fenton RA, Packer RK, Knepper MA. Long-term regulation of ENaC expression in kidney by angiotensin II. Hypertension 41: 1143–1150, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

