Effect of multivitamin supplementation on measles vaccine response among HIV-exposed uninfected Tanzanian infants
- PMID: 23720367
- PMCID: PMC3754503
- DOI: 10.1128/CVI.00183-13
Effect of multivitamin supplementation on measles vaccine response among HIV-exposed uninfected Tanzanian infants
Abstract
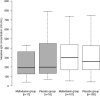
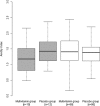
Immunization and nutritional interventions are mainstays of child health programs in sub-Saharan Africa, yet few published data exist on their interactions. HIV-exposed (but uninfected) infants enrolled in a randomized placebo-controlled trial of multivitamin supplements (vitamins B complex, C, and E) conducted in Tanzania were sampled for an assessment of measles IgG quantity and avidity at 15 to 18 months. Infants were vaccinated between 8.5 and 12 months of age, and all mothers received high-dose multivitamins as the standard of care. Of 201 HIV-exposed infants who were enrolled, 138 (68.7%) were seropositive for measles. There were no effects of infant multivitamin supplementation on measles seroconversion proportions, IgG concentrations, or IgG avidity (P > 0.05). The measles seroconversion proportion was greater for HIV-exposed infants vaccinated at 10 to 11 months of age than for those vaccinated at 8.5 to 10 months (P = 0.032) and greater for infants whose mothers had a CD4 T-cell count of <200 cells/μl than for infants whose mothers had a CD4 T-cell count of >350 cells/μl (P = 0.039). Stunted infants had a significantly decreased IgG quantity compared to nonstunted infants (P = 0.012). As for measles avidity, HIV-exposed infants vaccinated at 10 to 11 months had increased antibody avidity compared to those vaccinated at 8.5 to 10 months (P = 0.031). Maternal CD4 T-cell counts of <200 cells/μl were associated with decreased avidity compared to counts of >350 cells/μl (P = 0.047), as were lower infant height-for-age z-scores (P = 0.016). Supplementation with multivitamins containing B complex, C, and E does not appear to improve measles vaccine responses for HIV-exposed infants. Studies are needed to better characterize the impact of maternal HIV disease severity on the immune system development of HIV-exposed infants and the effect of malnutrition interventions on vaccine responses. (This study has been registered at ClinicalTrials.gov under registration no. NCT00197730.).
Figures
References
-
- Jones CE, Naidoo S, De Beer C, Esser M, Kampmann B, Hesseling AC. 2011. Maternal HIV infection and antibody responses against vaccine-preventable diseases in uninfected infants. JAMA 305:576–584 - PubMed
-
- Hygino J, Lima PG, Filho RG, Silva AA, Saramago CS, Andrade RM, Andrade DM, Andrade AF, Brindeiro R, Tanuri A, Bento CA. 2008. Altered immunological reactivity in HIV-1-exposed uninfected neonates. Clin. Immunol. 127:340–347 - PubMed
-
- Faye A, Pornprasert S, Mary JY, Dolcini G, Derrien M, Barré-Sinoussi F, Chaouat G, Menu EANRS 1267 Study Team, HIV-1 PMTCT-PlaNet 2007. Characterization of the main placental cytokine profiles from HIV-1-infected pregnant women treated with anti-retroviral drugs in France. Clin. Exp. Immunol. 149:430–439 - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

