Embryonic cardiac chamber maturation: Trabeculation, conduction, and cardiomyocyte proliferation
- PMID: 23720419
- PMCID: PMC3723796
- DOI: 10.1002/ajmg.c.31366
Embryonic cardiac chamber maturation: Trabeculation, conduction, and cardiomyocyte proliferation
Abstract
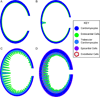
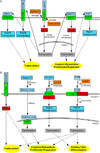
Congenital heart diseases are some of the most common human birth defects. Though some congenital heart defects can be surgically corrected, treatment options for other congenital heart diseases are very limited. In many congenital heart diseases, genetic defects lead to impaired embryonic heart development or growth. One of the key development processes in cardiac development is chamber maturation, and alterations in this maturation process can manifest as a variety of congenital defects including non-compaction, systolic dysfunction, diastolic dysfunction, and arrhythmia. During development, to meet the increasing metabolic demands of the developing embryo, the myocardial wall undergoes extensive remodeling characterized by the formation of muscular luminal protrusions called cardiac trabeculae, increased cardiomyocyte mass, and development of the ventricular conduction system. Though the basic morphological and cytological changes involved in early heart development are clear, much remains unknown about the complex biomolecular mechanisms governing chamber maturation. In this review, we highlight evidence suggesting that a wide variety of basic signaling pathways and biomechanical forces are involved in cardiac wall maturation.
Keywords: BMP; Ephrin; FGF; Left Ventricular Non-Compaction (LVNC); Neuregulin; Notch; Retinoic acid; Semaphorin; cardiac chamber maturation; cardiac trabeculation; cardiomyocyte proliferation; conduction; endothelin; extracellular matrix signaling.
Copyright © 2013 Wiley Periodicals, Inc.
Conflict of interest statement
None.
Figures

References
-
- Angelini A, Melacini P, Barbero F, Thiene G. Evolutionary persistence of spongy myocardium in humans. Circulation. 1999;99:2475–2475. - PubMed
-
- Beis D, Stainier DYR. In vivo cell biology: Following the zebrafish trend. Trends Cell Biol. 2006;16:105–112. - PubMed
-
- Berdougo E, Coleman H, Lee DH, Stainier DYR, Yelon D. Mutation of weak atrium/atrial myosin heavy chain disrupts atrial function and influences ventricular morphogenesis in zebrafish. Development. 2003;130:6121–6129. - PubMed
-
- Bhatia NL, Tajik AJ, Wilansky S, Steidley DE, Mookadam F. Isolated noncompaction of the left ventricular myocardium in adults: A systematic overview. J Card Fail. 2011;17:771–778. - PubMed
-
- Breckenridge RA, Anderson RH, Elliott PM. Isolated left ventricular non-compaction: The case for abnormal myocardial development. Cardiol Young. 2007;17:124–129. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

