ATP-binding cassette B10 regulates early steps of heme synthesis
- PMID: 23720443
- PMCID: PMC3817742
- DOI: 10.1161/CIRCRESAHA.113.301552
ATP-binding cassette B10 regulates early steps of heme synthesis
Abstract
Rationale: Heme plays a critical role in gas exchange, mitochondrial energy production, and antioxidant defense in cardiovascular system. The mitochondrial transporter ATP-binding cassette (ABC) B10 has been suggested to export heme out of the mitochondria and is required for normal hemoglobinization of erythropoietic cells and protection against ischemia-reperfusion injury in the heart; however, its primary function has not been established.
Objective: The aim of this study was to identify the function of ABCB10 in heme synthesis in cardiac cells.
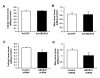
Methods and results: Knockdown of ABCB10 in cardiac myoblasts significantly reduced heme levels and the activities of heme-containing proteins, whereas supplementation with δ-aminolevulinic acid reversed these defects. Overexpression of mitochondrial δ-aminolevulinic acid synthase 2, the rate-limiting enzyme upstream of δ-aminolevulinic acid export, failed to restore heme levels in cells with ABCB10 downregulation. ABCB10 and heme levels were increased by hypoxia, and reversal of ABCB10 upregulation caused oxidative stress and cell death. Furthermore, ABCB10 knockdown in neonatal rat cardiomyocytes resulted in a significant delay of calcium removal from the cytoplasm, suggesting a relaxation defect. Finally, ABCB10 expression and heme levels were altered in failing human hearts and mice with ischemic cardiomyopathy.
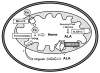
Conclusions: ABCB10 plays a critical role in heme synthesis pathway by facilitating δ-aminolevulinic acid production or export from the mitochondria. In contrast to previous reports, we show that ABCB10 is not a heme exporter and instead is required for the early mitochondrial steps of heme biosynthesis.
Keywords: ATP-binding cassette transporters; cardiomyopathies; heme; mitochondria; δ-aminolevulinic acid.
Figures





Similar articles
-
Reductions in the mitochondrial ABC transporter Abcb10 affect the transcriptional profile of heme biosynthesis genes.J Biol Chem. 2017 Sep 29;292(39):16284-16299. doi: 10.1074/jbc.M117.797415. Epub 2017 Aug 14. J Biol Chem. 2017. PMID: 28808058 Free PMC article.
-
Stimulation of the human mitochondrial transporter ABCB10 by zinc-mesoporphrin.PLoS One. 2020 Nov 30;15(11):e0238754. doi: 10.1371/journal.pone.0238754. eCollection 2020. PLoS One. 2020. PMID: 33253225 Free PMC article.
-
ATP Binding and Hydrolysis Properties of ABCB10 and Their Regulation by Glutathione.PLoS One. 2015 Jun 8;10(6):e0129772. doi: 10.1371/journal.pone.0129772. eCollection 2015. PLoS One. 2015. PMID: 26053025 Free PMC article.
-
Mitochondrial ABC transporters function: the role of ABCB10 (ABC-me) as a novel player in cellular handling of reactive oxygen species.Biochim Biophys Acta. 2012 Oct;1823(10):1945-57. doi: 10.1016/j.bbamcr.2012.07.013. Epub 2012 Aug 3. Biochim Biophys Acta. 2012. PMID: 22884976 Free PMC article. Review.
-
Intracellular peptide transporters in human--compartmentalization of the "peptidome".Pflugers Arch. 2007 Feb;453(5):591-600. doi: 10.1007/s00424-006-0083-4. Epub 2006 May 18. Pflugers Arch. 2007. PMID: 16710701 Review.
Cited by
-
Genomic adaptation of Ethiopian indigenous cattle to high altitude.Front Genet. 2022 Dec 9;13:960234. doi: 10.3389/fgene.2022.960234. eCollection 2022. Front Genet. 2022. PMID: 36568400 Free PMC article.
-
Cardiac PET Imaging of ATP Binding Cassette (ABC) Transporters: Opportunities and Challenges.Pharmaceuticals (Basel). 2023 Dec 11;16(12):1715. doi: 10.3390/ph16121715. Pharmaceuticals (Basel). 2023. PMID: 38139840 Free PMC article. Review.
-
A Recap of Heme Metabolism towards Understanding Protoporphyrin IX Selectivity in Cancer Cells.Int J Mol Sci. 2022 Jul 19;23(14):7974. doi: 10.3390/ijms23147974. Int J Mol Sci. 2022. PMID: 35887311 Free PMC article. Review.
-
Regulation of Heme Synthesis by Mitochondrial Homeostasis Proteins.Front Cell Dev Biol. 2022 Jun 27;10:895521. doi: 10.3389/fcell.2022.895521. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35832791 Free PMC article. Review.
-
ATP-binding cassette transporters mediate differential biosynthesis of glycosphingolipid species.J Lipid Res. 2021;62:100128. doi: 10.1016/j.jlr.2021.100128. Epub 2021 Sep 28. J Lipid Res. 2021. PMID: 34597626 Free PMC article.
References
-
- Harigae H, Furuyama K. Hereditary sideroblastic anemia: pathophysiology and gene mutations. Int J Hematol. 2010;92:425–431. - PubMed
-
- Fontenay M, Cathelin S, Amiot M, Gyan E, Solary E. Mitochondria in hematopoiesis and hematological diseases. Oncogene. 2006;25:4757–4767. - PubMed
-
- Ryter SW, Tyrrell RM. The heme synthesis and degradation pathways: role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radic Biol Med. 2000;28:289–309. - PubMed
-
- Worou ME, Belmokhtar K, Bonnet P, Vourc’h P, Machet MC, Khamis G, Eder V. Hemin decreases cardiac oxidative stress and fibrosis in a rat model of systemic hypertension via PI3K/Akt signalling. Cardiovasc Res. 2011;91:320–329. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

