Intranasal antibody gene transfer in mice and ferrets elicits broad protection against pandemic influenza
- PMID: 23720583
- PMCID: PMC4596530
- DOI: 10.1126/scitranslmed.3006299
Intranasal antibody gene transfer in mice and ferrets elicits broad protection against pandemic influenza
Abstract
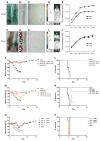
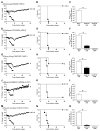
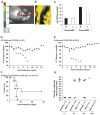
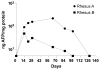
The emergence of a new influenza pandemic remains a threat that could result in a substantial loss of life and economic disruption worldwide. Advances in human antibody isolation have led to the discovery of monoclonal antibodies (mAbs) that have broad neutralizing activity against various influenza strains, although their direct use for prophylaxis is impractical. To overcome this limitation, our approach is to deliver antibody via adeno-associated virus (AAV) vectors to the site of initial infection, which, for respiratory viruses such as influenza, is the nasopharyngeal mucosa. AAV vectors based on serotype 9 were engineered to express a modified version of the previously isolated broadly neutralizing mAb to influenza A, FI6. We demonstrate that intranasal delivery of AAV9.FI6 into mice afforded complete protection and log reductions in viral load to 100 LD₅₀ (median lethal dose) of three clinical isolates of H5N1 and two clinical isolates of H1N1, all of which have been associated with historic human pandemics (including H1N1 1918). Similarly, complete protection was achieved in ferrets challenged with lethal doses of H5N1 and H1N1. This approach serves as a platform for the prevention of natural or deliberate respiratory diseases for which a protective antibody is available.
Conflict of interest statement
Figures
References
-
- Stöhr K. Influenza—WHO cares. Lancet Infect Dis. 2002;2:517. - PubMed
-
- Mak PW, Jayawardena S, Poon LL. The evolving threat of influenza viruses of animal origin and the challenges in developing appropriate diagnostics. Clin Chem. 2012;58:1527–1533. - PubMed
-
- Rappuoli R, Dormitzer PR. Influenza: Options to improve pandemic preparation. Science. 2012;336:1531–1533. - PubMed
-
- Corti D, Voss J, Gamblin SJ, Codoni G, Macagno A, Jarrossay D, Vachieri SG, Pinna D, Minola A, Vanzetta F, Silacci C, Fernandez-Rodriguez BM, Agatic G, Bianchi S, Giacchetto-Sasselli I, Calder L, Sallusto F, Collins P, Haire LF, Temperton N, Langedijk JP, Skehel JJ, Lanzavecchia A. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science. 2011;333:850–856. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

