Rethinking schizophrenia in the context of normal neurodevelopment
- PMID: 23720610
- PMCID: PMC3654207
- DOI: 10.3389/fncel.2013.00060
Rethinking schizophrenia in the context of normal neurodevelopment
Abstract
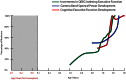
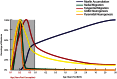
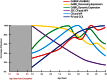
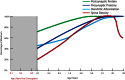
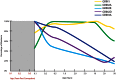
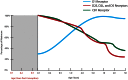
The schizophrenia brain is differentiated from the normal brain by subtle changes, with significant overlap in measures between normal and disease states. For the past 25 years, schizophrenia has increasingly been considered a neurodevelopmental disorder. This frame of reference challenges biological researchers to consider how pathological changes identified in adult brain tissue can be accounted for by aberrant developmental processes occurring during fetal, childhood, or adolescent periods. To place schizophrenia neuropathology in a neurodevelopmental context requires solid, scrutinized evidence of changes occurring during normal development of the human brain, particularly in the cortex; however, too often data on normative developmental change are selectively referenced. This paper focuses on the development of the prefrontal cortex and charts major molecular, cellular, and behavioral events on a similar time line. We first consider the time at which human cognitive abilities such as selective attention, working memory, and inhibitory control mature, emphasizing that attainment of full adult potential is a process requiring decades. We review the timing of neurogenesis, neuronal migration, white matter changes (myelination), and synapse development. We consider how molecular changes in neurotransmitter signaling pathways are altered throughout life and how they may be concomitant with cellular and cognitive changes. We end with a consideration of how the response to drugs of abuse changes with age. We conclude that the concepts around the timing of cortical neuronal migration, interneuron maturation, and synaptic regression in humans may need revision and include greater emphasis on the protracted and dynamic changes occurring in adolescence. Updating our current understanding of post-natal neurodevelopment should aid researchers in interpreting gray matter changes and derailed neurodevelopmental processes that could underlie emergence of psychosis.
Keywords: GABA receptor; NMDA receptor; cognition; dopamine receptor; excitatory synapses; myelination; neural migration; neurogenesis.
Figures
Similar articles
-
Schizophrenia: a tale of two critical periods for prefrontal cortical development.Transl Psychiatry. 2015 Aug 18;5(8):e623. doi: 10.1038/tp.2015.115. Transl Psychiatry. 2015. PMID: 26285133 Free PMC article. Review.
-
Brain structural and functional changes in adolescents with psychiatric disorders.Int J Adolesc Med Health. 2013;25(3):245-56. doi: 10.1515/ijamh-2013-0058. Int J Adolesc Med Health. 2013. PMID: 23828425 Free PMC article. Review.
-
Mechanisms contributing to prefrontal cortex maturation during adolescence.Neurosci Biobehav Rev. 2016 Nov;70:4-12. doi: 10.1016/j.neubiorev.2016.05.013. Epub 2016 May 24. Neurosci Biobehav Rev. 2016. PMID: 27235076 Free PMC article. Review.
-
The neurodevelopmental model of schizophrenia: update 2005.Mol Psychiatry. 2005 May;10(5):434-49. doi: 10.1038/sj.mp.4001642. Mol Psychiatry. 2005. PMID: 15700048 Review.
-
Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia.Schizophr Bull. 2005 Jul;31(3):672-96. doi: 10.1093/schbul/sbi034. Epub 2005 Jul 14. Schizophr Bull. 2005. PMID: 16020551 Review.
Cited by
-
The scheduling of adolescence with Netrin-1 and UNC5C.Elife. 2024 Jul 26;12:RP88261. doi: 10.7554/eLife.88261. Elife. 2024. PMID: 39056276 Free PMC article.
-
Effects of neonatal ethanol on cerebral cortex development through adolescence.Brain Struct Funct. 2019 Jun;224(5):1871-1884. doi: 10.1007/s00429-019-01881-1. Epub 2019 May 2. Brain Struct Funct. 2019. PMID: 31049690 Free PMC article.
-
Cortical abnormalities in youth at clinical high-risk for psychosis: Findings from the NAPLS2 cohort.Neuroimage Clin. 2019;23:101862. doi: 10.1016/j.nicl.2019.101862. Epub 2019 May 23. Neuroimage Clin. 2019. PMID: 31150956 Free PMC article.
-
Reciprocal causation models of cognitive vs volumetric cerebral intermediate phenotypes for schizophrenia in a pan-European twin cohort.Mol Psychiatry. 2015 Nov;20(11):1386-96. doi: 10.1038/mp.2014.152. Epub 2014 Dec 2. Mol Psychiatry. 2015. PMID: 25450228
-
Increased thin-spine density in frontal cortex pyramidal neurons in a genetic rat model of schizophrenia-relevant features.Eur Neuropsychopharmacol. 2021 Mar;44:79-91. doi: 10.1016/j.euroneuro.2021.01.006. Epub 2021 Jan 21. Eur Neuropsychopharmacol. 2021. PMID: 33485732 Free PMC article.
References
-
- Adriani W., Chiarotti F., Laviola G. (1998). Elevated novelty seeking and peculiar d-amphetamine sensitization in periadolescent mice compared with adult mice. Behav. Neurosci. 112, 1152–1166 - PubMed
-
- Akbarian S., Bunney W. E., Potkin S. G., Wigal S. B., Hagman J. O., Sandman C. A., et al. (1993). Altered distribution of nicotinamide-adenine dinucleotide phosphate diaphorase cells in frontal lobe of schizophrenics implies disturbances of cortical development. Arch. Gen. Psychiatry 50, 169–177 10.1001/archpsyc.1993.01820150007001 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

