Protection against lethal influenza with a viral mimic
- PMID: 23720727
- PMCID: PMC3719819
- DOI: 10.1128/JVI.01081-13
Protection against lethal influenza with a viral mimic
Abstract
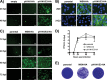
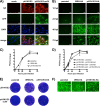
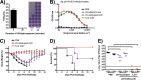
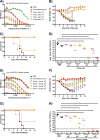
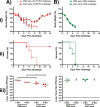
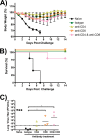
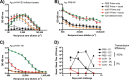
Despite countermeasures against influenza virus that prevent (vaccines) and treat (antivirals) infection, this upper respiratory tract human pathogen remains a global health burden, causing both seasonal epidemics and occasional pandemics. More potent and safe new vaccine technologies would contribute significantly to the battle against influenza and other respiratory infections. Using plasmid-based reverse genetics techniques, we have developed a single-cycle infectious influenza virus (sciIV) with immunoprotective potential. In our sciIV approach, the fourth viral segment, which codes for the receptor-binding and fusion protein hemagglutinin (HA), has been removed. Thus, upon infection of normal cells, although no infectious progeny are produced, the expression of other viral proteins occurs and is immunogenic. Consequently, sciIV is protective against influenza homologous and heterologous viral challenges in a mouse model. Vaccination with sciIV protects in a dose- and replication-dependent manner, which is attributed to both humoral responses and T cells. Safety, immunogenicity, and protection conferred by sciIV vaccination were also demonstrated in ferrets, where this immunization additionally blocked direct and aerosol transmission events. All together, our studies suggest that sciIV may have potential as a broadly protective vaccine against influenza virus.
Figures



References
-
- Wright PF, Neumann G, Kawaoka Y. 2007. Orthomyxoviruses, p 1691–1740 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed), Fields virology, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA
-
- Commission on Influenza, Board for the Investigation and Control of Influenza and Other Epidemic Diseases in the Army, Preventive Medicine Service, Office of the Surgeon General, United States Army 1944. A clinical evaluation of vaccination against influenza. Joint Report with Members of the Commission on Influenza, Board for the Investigation and Control of Influenza and Other Epidemic Diseases in the Army, Preventive Medicine Service, Office of the Surgeon General, United States Army. JAMA 124:982–985
-
- Osterholm MT, Kelley NS, Sommer A, Belongia EA. 2012. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect. Dis. 12:36–44 - PubMed
-
- Palese PS, Shaw ML. 2007. Orthomyxoviridae: the viruses and their replication, p 1647–1689 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed), Fields virology, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

