Accelerated development of pulmonary fibrosis via Cu,Zn-superoxide dismutase-induced alternative activation of macrophages
- PMID: 23720777
- PMCID: PMC3711337
- DOI: 10.1074/jbc.M112.410720
Accelerated development of pulmonary fibrosis via Cu,Zn-superoxide dismutase-induced alternative activation of macrophages
Abstract
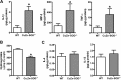
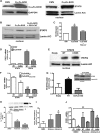
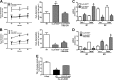
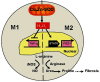
Macrophages not only initiate and accentuate inflammation after tissue injury, but they are also involved in resolution and repair. This difference in macrophage activity is the result of a differentiation process to either M1 or M2 phenotypes. M1 macrophages are pro-inflammatory and have microbicidal and tumoricidal activity, whereas the M2 macrophages are involved in tumor progression and tissue remodeling and can be profibrotic in certain conditions. Because mitochondrial Cu,Zn-superoxide dismutase (Cu,Zn-SOD)-mediated H2O2 is crucial for development of pulmonary fibrosis, we hypothesized that Cu,Zn-SOD modulated the macrophage phenotype. In this study, we demonstrate that Cu,Zn-SOD polarized macrophages to an M2 phenotype, and Cu,Zn-SOD-mediated H2O2 levels modulated M2 gene expression at the transcriptional level by redox regulation of a critical cysteine in STAT6. Furthermore, overexpression of Cu,Zn-SOD in mice resulted in a profibrotic environment and accelerated the development of pulmonary fibrosis, whereas polarization of macrophages to the M1 phenotype attenuated pulmonary fibrosis. Taken together, these observations provide a novel mechanism of Cu,Zn-SOD-mediated and Th2-independent M2 polarization and provide a potential therapeutic target for attenuating the accelerated development of pulmonary fibrosis.
Keywords: Hydrogen Peroxide; Macrophage Phenotype; Mitochondria; Oxidative Stress; Pulmonary Fibrosis; STAT6; Superoxide Dismutase (SOD).
Figures



References
-
- Selman M., King T. E., Pardo A. (2001) Idiopathic pulmonary fibrosis. Prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann. Intern Med. 134, 136–151 - PubMed
-
- Martinez F. O., Helming L., Gordon S. (2009) Alternative activation of macrophages. An immunologic functional perspective. Annu. Rev. Immunol. 27, 451–483 - PubMed
-
- Gordon S., Martinez F. O. (2010) Alternative activation of macrophages. Mechanism and functions. Immunity 32, 593–604 - PubMed
-
- Fridovich I., Freeman B. (1986) Antioxidant defenses in the lung. Annu. Rev. Physiol. 48, 693–702 - PubMed
-
- Okado-Matsumoto A., Fridovich I. (2001) Subcellular distribution of superoxide dismutases (SOD) in rat liver. Cu,Zn-SOD in mitochondria. J. Biol. Chem. 276, 38388–38393 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

