Epidermal growth factor receptor (EGFR) signaling promotes proliferation and survival in osteoprogenitors by increasing early growth response 2 (EGR2) expression
- PMID: 23720781
- PMCID: PMC3711314
- DOI: 10.1074/jbc.M112.447250
Epidermal growth factor receptor (EGFR) signaling promotes proliferation and survival in osteoprogenitors by increasing early growth response 2 (EGR2) expression
Abstract
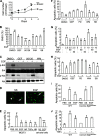
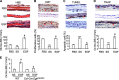
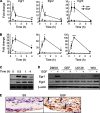
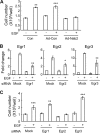
Maintaining bone architecture requires continuous generation of osteoblasts from osteoprogenitor pools. Our previous study of mice with epidermal growth factor receptor (EGFR) specifically inactivated in osteoblast lineage cells revealed that EGFR stimulates bone formation by expanding the population of mesenchymal progenitors. EGFR ligands are potent regulators for the osteoprogenitor pool, but the underlying mechanisms are largely unknown. Here we demonstrate that activation of EGFR increases the number of osteoprogenitors by promoting cell proliferation and suppressing either serum depletion-induced or TNFα-induced apoptosis mainly through the MAPK/ERK pathway. Mouse calvarial organ culture revealed that EGF elevated the number of proliferative cells and decreased the number of apoptotic cells, which led to increased osteoblasts. Microarray analysis of MC3T3 cells, an osteoprogenitor cell line, revealed that EGFR signaling stimulates the expression of MCL1, an antiapoptotic protein, and a family of EGR transcription factors (EGR1, -2, and -3). The up-regulation of MCL1 and EGR2 by EGF was further confirmed in osteoprogenitors close to the calvarial bone surface. Overexpression of NAB2, a co-repressor for EGRs, attenuated the EGF-induced increase in osteoprogenitor number. Interestingly, knocking down the expression of EGR2, but not EGR1 or -3, resulted in a similar effect. Using inhibitor, adenovirus overexpression, and siRNA approaches, we demonstrate that EGFR signaling activates the MAPK/ERK pathway to stimulate the expression of EGR2, which in turn leads to cell growth and MCL1-mediated cell survival. Taken together, our data clearly demonstrate that EGFR-induced EGR2 expression is critical for osteoprogenitor maintenance and new bone formation.
Keywords: Apoptosis; Bone; EGR2; Epidermal Growth Factor Receptor (EGFR); Osteoporosis; Osteoprogenitors; Proliferation.
Figures

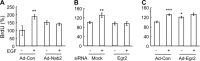
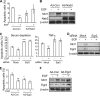
References
-
- Parfitt A. M., Han Z. H., Palnitkar S., Rao D. S., Shih M. S., Nelson D. (1997) Effects of ethnicity and age or menopause on osteoblast function, bone mineralization, and osteoid accumulation in iliac bone. J. Bone Miner. Res. 12, 1864–1873 - PubMed
-
- Bellantuono I., Aldahmash A., Kassem M. (2009) Aging of marrow stromal (skeletal) stem cells and their contribution to age-related bone loss. Biochim. Biophys. Acta 1792, 364–370 - PubMed
-
- Krampera M., Pasini A., Rigo A., Scupoli M. T., Tecchio C., Malpeli G., Scarpa A., Dazzi F., Pizzolo G., Vinante F. (2005) HB-EGF/HER-1 signaling in bone marrow mesenchymal stem cells: inducing cell expansion and reversibly preventing multilineage differentiation. Blood 106, 59–66 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

