The design and development of a high-throughput magneto-mechanostimulation device for cartilage tissue engineering
- PMID: 23721097
- PMCID: PMC3910453
- DOI: 10.1089/ten.TEC.2013.0225
The design and development of a high-throughput magneto-mechanostimulation device for cartilage tissue engineering
Abstract
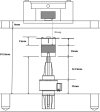
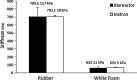
To recapitulate the in vivo environment and create neo-organoids that replace lost or damaged tissue requires the engineering of devices, which provide appropriate biophysical cues. To date, bioreactors for cartilage tissue engineering have focused primarily on biomechanical stimulation. There is a significant need for improved devices for articular cartilage tissue engineering capable of simultaneously applying multiple biophysical (electrokinetic and mechanical) stimuli. We have developed a novel high-throughput magneto-mechanostimulation bioreactor, capable of applying static and time-varying magnetic fields, as well as multiple and independently adjustable mechanical loading regimens. The device consists of an array of 18 individual stations, each of which uses contactless magnetic actuation and has an integrated Hall Effect sensing system, enabling the real-time measurements of applied field, force, and construct thickness, and hence, the indirect measurement of construct mechanical properties. Validation tests showed precise measurements of thickness, within 14 μm of gold standard calliper measurements; further, applied force was measured to be within 0.04 N of desired force over a half hour dynamic loading, which was repeatable over a 3-week test period. Finally, construct material properties measured using the bioreactor were not significantly different (p=0.97) from those measured using a standard materials testing machine. We present a new method for articular cartilage-specific bioreactor design, integrating combinatorial magneto-mechanostimulation, which is very attractive from functional and cost viewpoints.
Figures






Similar articles
-
[Research progress of bioreactor biophysical factors in cartilage tissue engineering].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2013 Jul;27(7):810-3. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2013. PMID: 24063168 Review. Chinese.
-
Concentric cylinder bioreactor for production of tissue engineered cartilage: effect of seeding density and hydrodynamic loading on construct development.Biotechnol Prog. 2003 Mar-Apr;19(2):510-21. doi: 10.1021/bp0256519. Biotechnol Prog. 2003. PMID: 12675595
-
Development and validation of a novel bioreactor system for load- and perfusion-controlled tissue engineering of chondrocyte-constructs.Biotechnol Bioeng. 2008 Nov 1;101(4):714-28. doi: 10.1002/bit.21955. Biotechnol Bioeng. 2008. PMID: 18814291
-
Design and validation of a bi-axial loading bioreactor for mechanical stimulation of engineered cartilage.Med Eng Phys. 2011 Jul;33(6):782-8. doi: 10.1016/j.medengphy.2011.01.013. Epub 2011 Feb 26. Med Eng Phys. 2011. PMID: 21356602
-
Hydrostatic pressure in articular cartilage tissue engineering: from chondrocytes to tissue regeneration.Tissue Eng Part B Rev. 2009 Mar;15(1):43-53. doi: 10.1089/ten.teb.2008.0435. Tissue Eng Part B Rev. 2009. PMID: 19196119 Free PMC article. Review.
Cited by
-
The Application of Bioreactors for Cartilage Tissue Engineering: Advances, Limitations, and Future Perspectives.Stem Cells Int. 2021 Jan 21;2021:6621806. doi: 10.1155/2021/6621806. eCollection 2021. Stem Cells Int. 2021. PMID: 33542736 Free PMC article. Review.
-
A Perfusion Bioreactor System for Cell Seeding and Oxygen-Controlled Cultivation of Three-Dimensional Cell Cultures.Tissue Eng Part C Methods. 2018 Oct;24(10):585-595. doi: 10.1089/ten.TEC.2018.0204. Tissue Eng Part C Methods. 2018. PMID: 30234443 Free PMC article.
-
Stimulation of chondrogenic differentiation of adult human bone marrow-derived stromal cells by a moderate-strength static magnetic field.Tissue Eng Part A. 2014 Jun;20(11-12):1612-20. doi: 10.1089/ten.tea.2013.0307. Epub 2014 Feb 7. Tissue Eng Part A. 2014. PMID: 24506272 Free PMC article.
References
-
- Schulz R.M., and Bader A.Cartilage tissue engineering and bioreactor systems for the cultivation and stimulation of chondrocytes. Eur Biophys J 36,539, 2007 - PubMed
-
- Bitton R.The economic burden of osteoarthritis. Am J Manag Care 15,S230, 2009 - PubMed
-
- Helmick C., Felson D., Lawrence R., Gabriel S., Hirsch R., Kwoh C.K., Liang M.H., Kremers H.M., Mayers M.D., Merkel P.A., Pillemer S.R., Reveille J.D., and Stone J.H.Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Arthritis Rheum 58,15, 2008 - PubMed
-
- Murphy L., and Helmick C.G.The impact of osteoarthritis in the United States: a population-health perspective. Am J Nurs 112,S13, 2012 - PubMed
-
- Korossis S.A., Bolland F., Kearney J.N., Fisher J., and Ingham E.Bioreactors in tissue engineering. In: Ashammakhi N., and Reis R.L., eds. Topics in Tissue Engineering, Volume 2, Chapter 8 2005, pp. 1–23 www.oulu.fi/spareparts/ebook_topics_in_t_e_vol2/abstracts/korossis_0102.pdf
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
