Hyaluronan fragments induce IFNβ via a novel TLR4-TRIF-TBK1-IRF3-dependent pathway
- PMID: 23721397
- PMCID: PMC3682892
- DOI: 10.1186/1476-9255-10-23
Hyaluronan fragments induce IFNβ via a novel TLR4-TRIF-TBK1-IRF3-dependent pathway
Abstract
Background: The extracellular matrix plays a critical role in insuring tissue integrity and water homeostasis. However, breakdown products of the extracellular matrix have emerged as endogenous danger signals, designed to rapidly activate the immune system against a potential pathogen breach. Type I interferons play a critical role in the immune response against viral infections. In the lungs, hylauronan (HA) exists as a high molecular weight, biologically inert extracellular matrix component that is critical for maintaining lung function. When lung tissue is injured, HA is broken down into lower molecular weight fragments that alert the immune system to the breach in tissue integrity by activating innate immune responses. HA fragments are known to induce inflammatory gene expression via TLR-MyD88-dependent pathways.
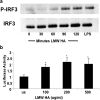
Methods: Primary peritoneal macrophages from C57BL/6 wild type, TLR4 null, TLR3 null, MyD88 null, and TRIF null mice as well as alveolar and peritoneal macrophage cell lines were stimulated with HA fragments and cytokine production was assessed by rt-PCR and ELISA. Western blot analysis for IRF3 was preformed on cell lysates from macrophages stimulate with HA fragments
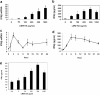
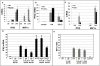
Results: We demonstrate for the first time that IFNβ is induced in murine macrophages by HA fragments. We also show that HA fragments induce IFNβ using a novel pathway independent of MyD88 but dependent on TLR4 via TRIF and IRF-3.
Conclusions: Overall our findings reveal a novel signaling pathway by which hyaluronan can modulate inflammation and demonstrate the ability of hyaluronan fragments to induce the expression of type I interferons in response to tissue injury even in the absence of viral infection. This is independent of the pathway of the TLR2-MyD88 used by these matrix fragments to induce inflammatory chemokines. Thus, LMW HA may be modifying the inflammatory milieu simultaneously via several pathways.
Figures

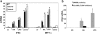
References
-
- Khaitov MR, Laza-Stanca V, Edwards MR, Walton RP, Rohde G, Contoli M, Papi A, Stanciu LA, Kotenko SV, Johnston SL. Respiratory virus induction of alpha-, beta- and lambda-interferons in bronchial epithelial cells and peripheral blood mononuclear cells. Allergy. 2009;64:375–386. doi: 10.1111/j.1398-9995.2008.01826.x. - DOI - PubMed
-
- Ashino S, Wakita D, Zhang Y, Chamoto K, Kitamura H, Nishimura T. CpG-ODN inhibits airway inflammation at effector phase through down-regulation of antigen-specific Th2-cell migration into lung. Int Immunol. 2008;20:259–266. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

