Accumulating Evidence and Research Organization (AERO) model: a new tool for representing, analyzing, and planning a translational research program
- PMID: 23721523
- PMCID: PMC3673838
- DOI: 10.1186/1745-6215-14-159
Accumulating Evidence and Research Organization (AERO) model: a new tool for representing, analyzing, and planning a translational research program
Abstract
Background: Maximizing efficiency in drug development is important for drug developers, policymakers, and human subjects. Limited funds and the ethical imperative of risk minimization demand that researchers maximize the knowledge gained per patient-subject enrolled. Yet, despite a common perception that the current system of drug development is beset by inefficiencies, there remain few approaches for systematically representing, analyzing, and communicating the efficiency and coordination of the research enterprise. In this paper, we present the first steps toward developing such an approach: a graph-theoretic tool for representing the Accumulating Evidence and Research Organization (AERO) across a translational trajectory.
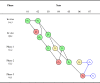
Methods: This initial version of the AERO model focuses on elucidating two dimensions of robustness: (1) the consistency of results among studies with an identical or similar outcome metric; and (2) the concordance of results among studies with qualitatively different outcome metrics. The visual structure of the model is a directed acyclic graph, designed to capture these two dimensions of robustness and their relationship to three basic questions that underlie the planning of a translational research program: What is the accumulating state of total evidence? What has been the translational trajectory? What studies should be done next?
Results: We demonstrate the utility of the AERO model with an application to a case study involving the antibacterial agent, moxifloxacin, for the treatment of drug-susceptible tuberculosis. We then consider some possible elaborations for the AERO model and propose a number of ways in which the tool could be used to enhance the planning, reporting, and analysis of clinical trials.
Conclusion: The AERO model provides an immediate visual representation of the number of studies done at any stage of research, depicting both the robustness of evidence and the relationship of each study to the larger translational trajectory. In so doing, it makes some of the invisible or inchoate properties of the research system explicit - helping to elucidate judgments about the accumulating state of evidence and supporting decision-making for future research.
Figures



References
-
- Tall AR, Yvan-Charvet L, Wang N. The Failure of Torcetrapib: Was it the molecule or the mechanism? Arteriosclerosis, Thrombosis, Vasc Biol. 2007;27:257–260. - PubMed
-
- Zhao L, Jin W, Rader D, Packard C, Feuerstein G. A Translational Medicine perspective of the development of torcetrapib: Does the failure of torcetrapib development cast a shadow on future development of lipid modifying agents, HDL elevation strategies or CETP as a viable molecular target for atherosclerosis? A case study of the use of biomarkers and Translational Medicine in atherosclerosis drug discovery and development. Biochem Pharmacol. 2009;78:315–325. - PubMed
-
- Arrowsmith J. Phase II failures: 2008-2010. Nat Rev Drug Discov. 2011;10:1. - PubMed
-
- Morgan P, Graaf PHVD, Arrowsmith J, Feltner DE, Drummond KS, Wegner CD, Street SDA. Can the flow of medicines be improved? Fundamental pharamacokinetic and pharmacological principles toward improving Phase II survival. Drug Discov Today. 2012;17:419–424. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

