Engineered SIRPα variants as immunotherapeutic adjuvants to anticancer antibodies
- PMID: 23722425
- PMCID: PMC3810306
- DOI: 10.1126/science.1238856
Engineered SIRPα variants as immunotherapeutic adjuvants to anticancer antibodies
Abstract
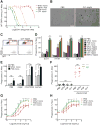
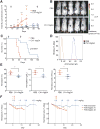
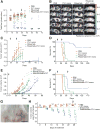
CD47 is an antiphagocytic signal that cancer cells employ to inhibit macrophage-mediated destruction. Here, we modified the binding domain of human SIRPα, the receptor for CD47, for use as a CD47 antagonist. We engineered high-affinity SIRPα variants with about a 50,000-fold increased affinity for human CD47 relative to wild-type SIRPα. As high-affinity SIRPα monomers, they potently antagonized CD47 on cancer cells but did not induce macrophage phagocytosis on their own. Instead, they exhibited remarkable synergy with all tumor-specific monoclonal antibodies tested by increasing phagocytosis in vitro and enhancing antitumor responses in vivo. This "one-two punch" directs immune responses against tumor cells while lowering the threshold for macrophage activation, thereby providing a universal method for augmenting the efficacy of therapeutic anticancer antibodies.
Figures

Comment in
-
Tumour immunology: cracking the combination.Nat Rev Immunol. 2013 Jul;13(7):469. doi: 10.1038/nri3481. Epub 2013 Jun 7. Nat Rev Immunol. 2013. PMID: 23743478 No abstract available.
-
Anticancer drugs: Cracking the combination.Nat Rev Drug Discov. 2013 Jul;12(7):505. doi: 10.1038/nrd4071. Nat Rev Drug Discov. 2013. PMID: 23812266 No abstract available.
-
Immunology. Making macrophages eat cancer.Science. 2013 Jul 5;341(6141):41-2. doi: 10.1126/science.1241716. Science. 2013. PMID: 23828933 No abstract available.
References
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646. - PubMed
-
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734. - PubMed
-
- Oldenborg PA, et al. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

