A human iPSC model of Ligase IV deficiency reveals an important role for NHEJ-mediated-DSB repair in the survival and genomic stability of induced pluripotent stem cells and emerging haematopoietic progenitors
- PMID: 23722522
- PMCID: PMC3705601
- DOI: 10.1038/cdd.2013.44
A human iPSC model of Ligase IV deficiency reveals an important role for NHEJ-mediated-DSB repair in the survival and genomic stability of induced pluripotent stem cells and emerging haematopoietic progenitors
Abstract
DNA double strand breaks (DSBs) are the most common form of DNA damage and are repaired by non-homologous-end-joining (NHEJ) or homologous recombination (HR). Several protein components function in NHEJ, and of these, DNA Ligase IV is essential for performing the final 'end-joining' step. Mutations in DNA Ligase IV result in LIG4 syndrome, which is characterised by growth defects, microcephaly, reduced number of blood cells, increased predisposition to leukaemia and variable degrees of immunodeficiency. In this manuscript, we report the creation of a human induced pluripotent stem cell (iPSC) model of LIG4 deficiency, which accurately replicates the DSB repair phenotype of LIG4 patients. Our findings demonstrate that impairment of NHEJ-mediated-DSB repair in human iPSC results in accumulation of DSBs and enhanced apoptosis, thus providing new insights into likely mechanisms used by pluripotent stem cells to maintain their genomic integrity. Defects in NHEJ-mediated-DSB repair also led to a significant decrease in reprogramming efficiency of human cells and accumulation of chromosomal abnormalities, suggesting a key role for NHEJ in somatic cell reprogramming and providing insights for future cell based therapies for applications of LIG4-iPSCs. Although haematopoietic specification of LIG4-iPSC is not affected per se, the emerging haematopoietic progenitors show a high accumulation of DSBs and enhanced apoptosis, resulting in reduced numbers of mature haematopoietic cells. Together our findings provide new insights into the role of NHEJ-mediated-DSB repair in the survival and differentiation of progenitor cells, which likely underlies the developmental abnormalities observed in many DNA damage disorders. In addition, our findings are important for understanding how genomic instability arises in pluripotent stem cells and for defining appropriate culture conditions that restrict DNA damage and result in ex vivo expansion of stem cells with intact genomes.
Figures
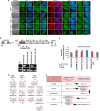
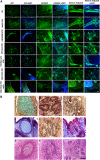
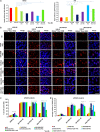

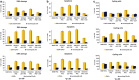
Comment in
-
Reprogramming and genome integrity: role of non-homologous end joining.Cell Death Differ. 2013 Oct;20(10):1285-6. doi: 10.1038/cdd.2013.95. Cell Death Differ. 2013. PMID: 24013776 Free PMC article. No abstract available.
References
-
- Jeggo PA. DNA breakage and repair. Adv Genet. 1998;38:185–218. - PubMed
-
- Holthausen JT, Wyman C, Kanaar R. Regulation of DNA strand exchange in homologous recombination. DNA Repair (Amst) 2010;9:1264–1272. - PubMed
-
- Lieber MR, Ma Y, Pannicke U, Schwarz K. Mechanism and regulation of human non-homologous DNA end-joining. Nat Rev Mol Cell Biol. 2003;4:712–720. - PubMed
-
- Grawunder U, Grawunder U, Wilm M, Wu M, Kulesza P, Wilson TE, et al. Activity of DNA ligase IV stimulated by complex formation with XRCC4 protein in mammalian cells. Nature. 1997;388:492–495. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

