Frameshifting dynamics
- PMID: 23722586
- PMCID: PMC4011568
- DOI: 10.1002/bip.22293
Frameshifting dynamics
Erratum in
- Biopolymers. 2014 Mar;101(3):306
Abstract
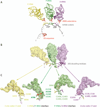
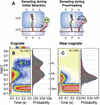
Translation of messenger RNA by a ribosome occurs three nucleotides at a time from start signal to stop. However, a frameshift means that some nucleotides are read twice or some are skipped, and the following sequence of amino acids is completely different from the sequence in the original frame. In some messenger RNAs, including viral RNAs, frameshifting is programmed with RNA signals to produce specific ratios of proteins vital to the replication of the organism. The mechanisms that cause frameshifting have been studied for many years, but there are no definitive conclusions. We review ribosome structure and dynamics in relation to frameshifting dynamics provided by classical ensemble studies, and by new single-molecule methods using optical tweezers and FRET.
Keywords: FRET; fluorescence; optical tweezers; programmed frameshifting; ribosome structure; single molecule; translation.
Copyright © 2013 Wiley Periodicals, Inc.
Figures




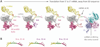




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

