Kindlin-2 mediates activation of TGF-β/Smad signaling and renal fibrosis
- PMID: 23723426
- PMCID: PMC3752947
- DOI: 10.1681/ASN.2012101041
Kindlin-2 mediates activation of TGF-β/Smad signaling and renal fibrosis
Abstract
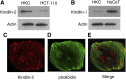
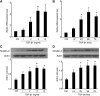
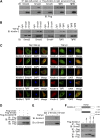
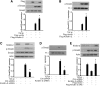
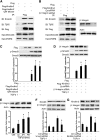
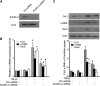
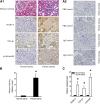
Activation of TGF-β/Smad signaling plays a central role in the pathogenesis of tubulointerstitial fibrosis, but the mechanisms underlying the initial interaction of the TGF-β receptor with Smads, leading to their activation, remain unclear. Here, we found that Kindlin-2, an integrin-binding protein, physically mediated the interaction of the TGF-β type I receptor (TβRI) with Smad3 in human kidney tubular epithelial cells. Kindlin-2 bound to TβRI through its FERM domain and to Smad3 through its N terminus. Overexpression of Kindlin-2 increased TGF-β-induced Smad3 activation. Knockdown of Kindlin-2 significantly suppressed the engagement of TβRI with Smad3 and inhibited TGF-β-induced Smad3 activation, as well as the expression of its target genes. Neither transfection of a Kindlin-2 mutant incapable of binding to β1 integrin nor knockdown of β1 integrin influenced the effect of Kindlin-2 on TGF-β1-induced Smad3 activation, indicating that this effect is independent of integrin. Kindlin-2 expression was markedly increased, predominantly in renal tubular epithelial cells, both in the unilateral ureteral obstruction model of kidney fibrosis and in human tissue exhibiting tubulointerstitial fibrosis. Furthermore, in the unilateral ureteral obstruction model, knocking down Kindlin-2 significantly inhibited activation of TGF-β/Smad signaling, decreased the expression of matrix genes, and ameliorated fibrosis. In summary, Kindlin-2 physically interacts with both TβRI and Smad3, promoting the activation of TGF-β/Smad signaling and contributing to the pathogenesis of tubulointerstitial fibrosis. Blockade of Kindlin-2 might be a rational therapeutic strategy for the treatment of fibrotic kidney diseases.
Figures


Comment in
-
Kindlin-2: a new player in renal fibrogenesis.J Am Soc Nephrol. 2013 Sep;24(9):1339-40. doi: 10.1681/ASN.2013060627. Epub 2013 Jul 11. J Am Soc Nephrol. 2013. PMID: 23847279 Free PMC article. No abstract available.
References
-
- Zeisberg M, Neilson EG: Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol 21: 1819–1834, 2010 - PubMed
-
- Nangaku M: Mechanisms of tubulointerstitial injury in the kidney: Final common pathways to end-stage renal failure. Intern Med 43: 9–17, 2004 - PubMed
-
- Boor P, Ostendorf T, Floege J: Renal fibrosis: Novel insights into mechanisms and therapeutic targets. Nat Rev Nephrol 6: 643–656, 2010 - PubMed
-
- Okada H, Kalluri R: Cellular and molecular pathways that lead to progression and regression of renal fibrogenesis. Curr Mol Med 5: 467–474, 2005 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

