2-[(5'-Chloro-1,1':3',1''-terphenyl-4'-yl)imino]-acenaphthylen-1(2H)-one
- PMID: 23723792
- PMCID: PMC3647826
- DOI: 10.1107/S1600536813008015
2-[(5'-Chloro-1,1':3',1''-terphenyl-4'-yl)imino]-acenaphthylen-1(2H)-one
Abstract
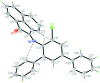
The title compound, C30H18ClNO, is a product of the condensation reaction of acenaphthyl-ene-1,2-dione and 5'-chloro-1,1':3',1''-terphenyl-4'-amine. The acenaphthyl-ene fragment and two terminal phenyl rings are rotated relative to the central benzene ring by 72.2 (3), 43.2 (3) and 41.2 (3)°, respectively. This mol-ecular conformation is supported by weak C-H⋯π inter-actions. In the crystal, mol-ecules form centrosymmetric dimers by the stacking inter-actions between two neighboring acenaphthyl-ene fragments, with an inter-planar distance of 3.365 (3) Å. The dimers are bound to each other by weak C-H⋯N and C-H⋯π inter-actions, forming a three-dimensional framework.
Figures

References
-
- Agilent (2012). CrysAlis PRO Agilent Technologies, Yarnton, England.
-
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J Appl. Cryst 42, 339–341.
-
- Higuchi, M., Shiki, S., Ariga, K. & Yamamoto, K. (2001). J. Am. Chem. Soc. 123, 4414–4420. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
