Mover is a homomeric phospho-protein present on synaptic vesicles
- PMID: 23723986
- PMCID: PMC3665746
- DOI: 10.1371/journal.pone.0063474
Mover is a homomeric phospho-protein present on synaptic vesicles
Abstract
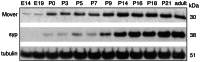
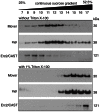
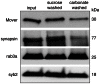
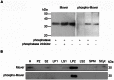
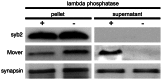
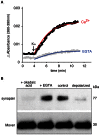
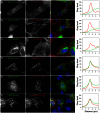
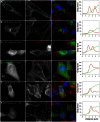
With remarkably few exceptions, the molecules mediating synaptic vesicle exocytosis at active zones are structurally and functionally conserved between vertebrates and invertebrates. Mover was found in a yeast-2-hybrid assay using the vertebrate-specific active zone scaffolding protein bassoon as a bait. Peptides of Mover have been reported in proteomics screens for self-interacting proteins, phosphorylated proteins, and synaptic vesicle proteins, respectively. Here, we tested the predictions arising from these screens. Using flotation assays, carbonate stripping of peripheral membrane proteins, mass spectrometry, immunogold labelling of purified synaptic vesicles, and immuno-organelle isolation, we found that Mover is indeed a peripheral synaptic vesicle membrane protein. In addition, by generating an antibody against phosphorylated Mover and Western blot analysis of fractionated rat brain, we found that Mover is a bona fide phospho-protein. The localization of Mover to synaptic vesicles is phosphorylation dependent; treatment with a phosphatase caused Mover to dissociate from synaptic vesicles. A yeast-2-hybrid screen, co-immunoprecipitation and cell-based optical assays of homomerization revealed that Mover undergoes homophilic interaction, and regions within both the N- and C- terminus of the protein are required for this interaction. Deleting a region required for homomeric interaction abolished presynaptic targeting of recombinant Mover in cultured neurons. Together, these data prove that Mover is associated with synaptic vesicles, and implicate phosphorylation and multimerization in targeting of Mover to synaptic vesicles and presynaptic sites.
Conflict of interest statement
Figures



References
-
- Sudhof TC (2004) The synaptic vesicle cycle. Annual review of neuroscience 27: 509–547. - PubMed
-
- Chapman ER (2008) How does synaptotagmin trigger neurotransmitter release? Annual review of biochemistry 77: 615–641. - PubMed
-
- Augustin I, Rosenmund C, Sudhof TC, Brose N (1999) Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature 400: 457–461. - PubMed
-
- Siksou L, Varoqueaux F, Pascual O, Triller A, Brose N, et al. (2009) A common molecular basis for membrane docking and functional priming of synaptic vesicles. The European journal of neuroscience 30: 49–56. - PubMed
-
- Verhage M, Maia AS, Plomp JJ, Brussaard AB, Heeroma JH, et al. (2000) Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science 287: 864–869. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

