Two new cave-dwelling species of the short-tailed Whipscorpion genus Rowlandius (Arachnida: Schizomida: Hubbardiidae) from northeastern Brazil, with comments on male dimorphism
- PMID: 23723989
- PMCID: PMC3661548
- DOI: 10.1371/journal.pone.0063616
Two new cave-dwelling species of the short-tailed Whipscorpion genus Rowlandius (Arachnida: Schizomida: Hubbardiidae) from northeastern Brazil, with comments on male dimorphism
Abstract
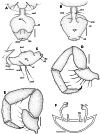
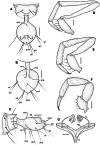
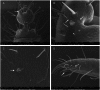
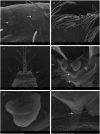
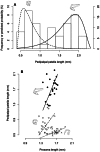
Two new species of the arachnid order Schizomida, Rowlandius ubajara sp.nov. and Rowlandius potiguar sp.nov., are described based on both male and female specimens collected in caves from northeastern Brazil. Rowlandius ubajara is known only from the Ubajara Cave, in the state of Ceará; R. potiguar is recorded from 20 caves of the Apodi Limestone Group, in the state of Rio Grande do Norte. A remarkable dimorphism in male pedipalp length is described and analyzed in R. potiguar. The distribution of male pedipalp length is clearly bimodal in the species, but the two male morphs (homeomorphic and heteromorphic) present some overlap in the sizes of this structure. Moreover, males show a steeper allometry in pedipalp length than females, indicating that this trait is under a different selective regime in males and in females.
Conflict of interest statement
Figures

References
-
- Tourinho ALM, Kury AB (1999) The Southernmost record of Schizomida in South America, first record of Schizomida for Rio de Janeiro and of Stenochrus Chamberlin, 1922 for Brazil (Arachnida, Schizomida, Hubbardiidae). Boletim do Museu Nacional, Zoologia 405: 1–6.
-
- Santos AJ, Dias SC, Brescovit AD, Santos PP (2008) The arachnid order Schizomida in the Brazilian Atlantic Forest: a new species of Rowlandius and new records of Stenochrus portoricensis (Schizomida: Hubbardiidae). Zootaxa 1850: 53–60.
-
- Reddell JR, Cokendolpher JC (1995) Catalogue, bibliography and generic revision of the order Schizomida (Arachnida). Texas Memorial Museum, Speleological Monographs 4: 1–170.
-
- Auler A, Rubbioli E, Brandi R (2001) As grandes cavernas do Brasil. Belo Horizonte: Grupo Bambuí de Pesquisas Espeleológicas. 228 p.
-
- Ferreira RL, Souza-Silva M, Oliveira-Bernardi LF (2009) Contexto bioespeleológico. In: Drummond GM, Martins CS, Greco MB, Vieira F, editors. Biota Minas: diagnóstico do conhecimento sobre a biodiversidade no estado de Minas Gerais – subsídio para o programa Biota Minas. Belo Horizonte: Fundação Biodiversitas. 160–175.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

