Systematic review of antiretroviral-associated lipodystrophy: lipoatrophy, but not central fat gain, is an antiretroviral adverse drug reaction
- PMID: 23723990
- PMCID: PMC3665842
- DOI: 10.1371/journal.pone.0063623
Systematic review of antiretroviral-associated lipodystrophy: lipoatrophy, but not central fat gain, is an antiretroviral adverse drug reaction
Abstract
Background: Lipoatrophy and/or central fat gain are observed frequently in patients on antiretroviral therapy (ART). Both are assumed to be antiretroviral adverse drug reactions.
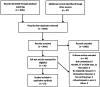
Methods: We conducted a systematic review to determine whether fat loss or gain was more common in HIV-infected patients on ART than in uninfected controls; was associated with specific antiretrovirals; and would reverse after switching antiretrovirals.
Results: Twenty-seven studies met our inclusion criteria. One cohort study reported more lipoatrophy, less subcutaneous fat gain, but no difference in central fat gain in HIV-infected patients on ART than in controls. Randomised controlled trials (RCTs) showed more limb fat loss (or less fat gain) with the following regimens: stavudine (versus other nucleoside reverse transcriptase inhibitors (NRTIs)); efavirenz (versus protease inhibitors (PIs)); and NRTI-containing (versus NRTI-sparing). RCTs showed increased subcutaneous fat after switching to NRTI-sparing regimens or from stavudine/zidovudine to abacavir/tenofovir. There were no significant between-group differences in trunk and/or visceral fat gain in RCTs of various regimens, but results from efavirenz versus PI regimens were inconsistent. There was no significant between-group differences in central fat gain in RCTs switched to NRTI-sparing regimens, or from PI-containing regimens.
Conclusions: There is clear evidence of a causal relationship between NRTIs (especially thymidine analogues) and lipoatrophy, with concomitant PIs possibly having an ameliorating effect or efavirenz causing additive toxicity. By contrast, central fat gain appears to be a consequence of treating HIV infection, because it is not different from controls, is not linked to any antiretroviral class, and doesn't improve on switching.
Conflict of interest statement
References
-
- Carr A (2003) HIV lipodystrophy: risk factors, pathogenesis, diagnosis and management. AIDS 17: S141–S148. - PubMed
-
- Grinspoon S, Carr A (2005) Cardiovascular Risk and Body-Fat Abnormalities in HIV-Infected Adults. N Engl J Med 352: 48–62. - PubMed
-
- Negredo E, Miro O, Rodriguez-Santiago B, Garrabou G, Estany C, et al. (2009) Improvement of mitochondrial toxicity in patients receiving a nucleoside reverse-transcriptase inhibitor-sparing strategy: results from the Multicenter Study with Nevirapine and Kaletra (MULTINEKA). Clin Infect Dis 49: 892–900. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous