The role of paracrine and autocrine signaling in the early phase of adipogenic differentiation of adipose-derived stem cells
- PMID: 23723991
- PMCID: PMC3665830
- DOI: 10.1371/journal.pone.0063638
The role of paracrine and autocrine signaling in the early phase of adipogenic differentiation of adipose-derived stem cells
Abstract
Introduction: High cell density is known to enhance adipogenic differentiation of mesenchymal stem cells, suggesting secretion of signaling factors or cell-contact-mediated signaling. By employing microfluidic biochip technology, we have been able to separate these two processes and study the secretion pathways.
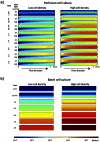
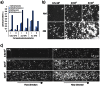
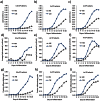
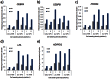
Methods and results: Adipogenic differentiation of human adipose-derived stem cells (ASCs) cultured in a microfluidic system was investigated under perfusion conditions with an adipogenic medium or an adipogenic medium supplemented with supernatant from differentiating ASCs (conditioned medium). Conditioned medium increased adipogenic differentiation compared to adipogenic medium with respect to accumulation of lipid-filled vacuoles and gene expression of key adipogenic markers (C/EBPα, C/EBPβ, C/EBPδ, PPARγ, LPL and adiponectin). The positive effects of conditioned medium were observed early in the differentiation process.
Conclusions: Using different cell densities and microfluidic perfusion cell cultures to suppress the effects of cell-released factors, we have demonstrated the significant role played by auto- or paracrine signaling in adipocyte differentiation. The cell-released factor(s) were shown to act in the recruitment phase of the differentiation process.
Conflict of interest statement
Figures


References
-
- Koppen A, Kalkhoven E (2010) Brown vs white adipocytes: The PPAR gamma coregulator story. Febs Letters 584: 3250–3259. - PubMed
-
- Rosen ED, MacDougald OA (2006) Adipocyte differentiation from the inside out. Nature Reviews Molecular Cell Biology 7: 885–896. - PubMed
-
- Mizuno H, Tobita M, Uysal AC (2012) Concise Review: Adipose-Derived Stem Cells as a Novel Tool for Future Regenerative Medicine. Stem Cells 30: 804–810. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

