Phase 1b randomized trial and follow-up study in Uganda of the blood-stage malaria vaccine candidate BK-SE36
- PMID: 23724021
- PMCID: PMC3665850
- DOI: 10.1371/journal.pone.0064073
Phase 1b randomized trial and follow-up study in Uganda of the blood-stage malaria vaccine candidate BK-SE36
Abstract
Background: Up to now a malaria vaccine remains elusive. The Plasmodium falciparum serine repeat antigen-5 formulated with aluminum hydroxyl gel (BK-SE36) is a blood-stage malaria vaccine candidate that has undergone phase 1a trial in malaria-naive Japanese adults. We have now assessed the safety and immunogenicity of BK-SE36 in a malaria endemic area in Northern Uganda.
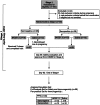
Methods: We performed a two-stage, randomized, single-blinded, placebo-controlled phase 1b trial (Current Controlled trials ISRCTN71619711). A computer-generated sequence randomized healthy subjects for 2 subcutaneous injections at 21-day intervals in Stage1 (21-40 year-olds) to 1-mL BK-SE36 (BKSE1.0) (n = 36) or saline (n = 20) and in Stage2 (6-20 year-olds) to BKSE1.0 (n = 33), 0.5-mL BK-SE36 (BKSE0.5) (n = 33), or saline (n = 18). Subjects and laboratory personnel were blinded. Safety and antibody responses 21-days post-second vaccination (Day42) were assessed. Post-trial, to compare the risk of malaria episodes 130-365 days post-second vaccination, Stage2 subjects were age-matched to 50 control individuals.
Results: Nearly all subjects who received BK-SE36 had induration (Stage1, n = 33, 92%; Stage2, n = 63, 96%) as a local adverse event. No serious adverse event related to BK-SE36 was reported. Pre-existing anti-SE36 antibody titers negatively correlated with vaccination-induced antibody response. At Day42, change in antibody titers was significant for seronegative adults (1.95-fold higher than baseline [95% CI, 1.56-2.43], p = 0.004) and 6-10 year-olds (5.71-fold [95% CI, 2.38-13.72], p = 0.002) vaccinated with BKSE1.0. Immunogenicity response to BKSE0.5 was low and not significant (1.55-fold [95% CI, 1.24-1.94], p = 0.75). In the ancillary analysis, cumulative incidence of first malaria episodes with ≥5000 parasites/µL was 7 cases/33 subjects in BKSE1.0 and 10 cases/33 subjects in BKSE0.5 vs. 29 cases/66 subjects in the control group. Risk ratio for BKSE1.0 was 0.48 (95% CI, 0.24-0.98; p = 0.04).
Conclusion: BK-SE36 is safe and immunogenic. The promising potential of BK-SE36, observed in the follow-up study, warrants a double-blind phase 1/2b trial in children under 5 years.
Trial registration: Controlled-Trials.com ISRCTN71619711.
Conflict of interest statement
Figures


References
-
- Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, et al. (2012) Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet 379: 413–431. - PubMed
-
- Greenwood BM, Targett GA (2011) Malaria vaccines and the new malaria agenda. Clin Microbiol Infect 17: 1600–1607. - PubMed
-
- Agnandji ST, Lell B, Soulanoudjingar SS, Fernandes JF, Abossolo BP, et al. (2011) First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med 365: 1863–1875. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

