Clinical and immunological changes of immunotherapy in patients with atopic dermatitis: randomized controlled trial
- PMID: 23724240
- PMCID: PMC3658480
- DOI: 10.5402/2012/183983
Clinical and immunological changes of immunotherapy in patients with atopic dermatitis: randomized controlled trial
Abstract
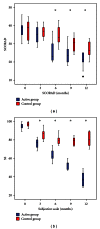
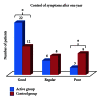
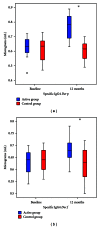
Background. Immunotherapy has proven to be an useful tool in the management of allergic respiratory diseases; however, little has been studied in atopic dermatitis. Objective. To evaluate the clinical and immunological impact of immunotherapy with mites allergen extracts in atopic dermatitis. Methods. Patients with atopic dermatitis were assigned with computer-generated randomization to either of the following groups: (a) controls received only topical treatment with steroids and/or tacrolimus and (b) actively treated patients received topical treatment plus immunotherapy. Levels of serum total IgE, mites-specific IgE and IgG4 were assessed at study start and after one year of immunotherapy. Results. 31 patients in the active group and 29 in the control group completed the study. Symptoms and medication scores were significantly reduced in the active group after six months. Three patients in the control group showed new sensitizations to mites, while 3 patients in the active group showed neosensitization to shrimp with negative oral food challenge. We observed significant increase of mites-specific IgG4 levels in active group. Conclusion. Specific allergen immunotherapy induced a tolerogenic IgG4 response to mite allergens associated with favorable clinical effects in atopic dermatitis patients.
Figures



References
-
- Bieber T. Atopic dermatitis. New England Journal of Medicine. 2008;358(14):1483–1494. - PubMed
-
- Bousquet J, Lockey R, Malling HJ, et al. Allergen immunotherapy: therapeutic vaccines for allergic diseases—A WHO position paper. Journal of Allergy and Clinical Immunology. 1998;102(4):558–562. - PubMed
-
- Pajno GB, Caminiti L, Vita D, et al. Sublingual immunotherapy in mite-sensitized children with atopic dermatitis: a randomized, double-blind, placebo-controlled study. Journal of Allergy and Clinical Immunology. 2007;120(1):164–170. - PubMed
-
- Bussmann C, Böckenhoff A, Henke H, Werfel T, Novak N. Does allergen-specific immunotherapy represent a therapeutic option for patients with atopic dermatitis? Journal of Allergy and Clinical Immunology. 2006;118(6):1292–1298. - PubMed
LinkOut - more resources
Full Text Sources

