Antinociceptive Activity and Redox Profile of the Monoterpenes (+)-Camphene, p-Cymene, and Geranyl Acetate in Experimental Models
- PMID: 23724298
- PMCID: PMC3658412
- DOI: 10.1155/2013/459530
Antinociceptive Activity and Redox Profile of the Monoterpenes (+)-Camphene, p-Cymene, and Geranyl Acetate in Experimental Models
Abstract
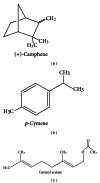
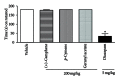
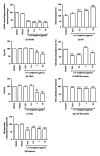
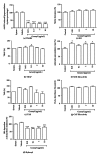
Objective. To evaluate antinocicpetive and redox properties of the monoterpenes (+)-camphene, p-cymene, and geranyl acetate in in vivo and in vitro experimental models. Methods. Evaluation of the in vitro antioxidant activity of (+)-camphene, p-cymene, and geranyl acetate using different free radical-generating systems and evaluation of antinociceptive actions by acetic acid-induced writhing and formalin-induced nociception tests in mice. Results. p-Cymene has the strongest antinociceptive effect, but (+)-camphene and geranyl acetate also present significant activity at high doses (200 mg/kg). (+)-Camphene had the strongest antioxidant effect in vitro at TBARS and TRAP/TAR assays and also had the highest scavenging activities against different free radicals, such as hydroxyl and superoxide radicals. Sodium nitroprussiate-derived NO production was enhanced by (+)-camphene. Geranyl acetate and p-cymene also presented some antioxidant effects, but with a varying profile according the free radical-generating system studied. Conclusion. (+)-Camphene, p-cymene, and geranyl acetate may present pharmacological properties related to inflammation and pain-related processes, being potentially useful for development of new therapeutic strategies, with limited possibilities for p-cymene and geranyl acetate.
Figures

References
-
- Calixto JB. Twenty-five years of research on medicinal plants in Latin America: a personal view. Journal of Ethnopharmacology. 2005;100(1-2):131–134. - PubMed
-
- Phillipson JD. Phytochemistry and pharmacognosy. Phytochemistry. 2007;68(22–24):2960–2972. - PubMed
-
- Guimarães AG, Oliveira GF, Melo MS, et al. Bioassay-guided evaluation of antioxidant and antinociceptive activities of carvacrol. Basic and Clinical Pharmacology and Toxicology. 2010;107(6):949–957. - PubMed
-
- Figueirinha A, Cruz MT, Francisco V, Lopes MC, Batista MT. Anti-inflammatory activity of cymbopogon citratus leaf infusion in lipopolysaccharide-stimulated dendritic cells: contribution of the polyphenols. Journal of Medicinal Food. 2010;13(3):681–690. - PubMed
-
- Orhan I, Kartal M, Kan Y, Şener B. Activity of essential oils and individual components against acetyl-and butyrylcholinesterase. Zeitschrift fur Naturforschung C. 2008;63(7-8):547–553. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

