Differential modulation of nociceptive versus non-nociceptive synapses by endocannabinoids
- PMID: 23725095
- PMCID: PMC3693973
- DOI: 10.1186/1744-8069-9-26
Differential modulation of nociceptive versus non-nociceptive synapses by endocannabinoids
Abstract
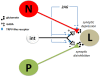
Background: Although a number of clinical and preclinical studies have demonstrated analgesic effects of cannabinoid treatments, there are also instances when cannabinoids have had no effect or even exacerbated pain. The observed pro-nociceptive effects appear to be due to cannabinoid-induced disinhibition of afferent synaptic input to nociceptive circuits. To better understand how cannabinoid-mediated plasticity can have both pro- and anti-nociceptive effects, we examined the possibility that cannabinoids differentially modulate nociceptive vs. non-nociceptive synapses onto a shared postsynaptic target. These experiments were carried out in the central nervous system (CNS) of the medicinal leech, in which it is possible to intracellularly record from presynaptic nociceptive (N-cell) or pressure-sensitive (P-cell) neurons and their shared postsynaptic targets.
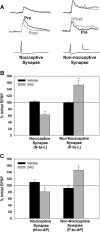
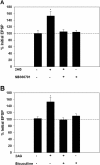
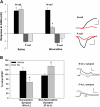
Results: The endocannabinoid 2-arachidonoyl glycerol (2AG) elicited significant long-lasting depression in nociceptive (N-cell) synapses. However, non-nociceptive (P-cell) synapses were potentiated following 2AG treatment. 2AG-induced potentiation of non-nociceptive synapses was blocked by the TRPV antagonist SB366791, suggesting involvement of the same TRPV-like receptor that has already been shown to mediate endocannabinoid-dependent depression in nociceptive inputs. Treatment with the GABA receptor antagonist bicuculline also blocked 2AG-induced potentiation, consistent with the idea that increased synaptic signaling was the result of endocannabinoid-mediated disinhibition. Interestingly, while bicuculline by itself increased non-nociceptive synaptic transmission, nociceptive synapses were depressed by this GABA receptor antagonist indicating that nociceptive synapses were actually excited by GABAergic input. Consistent with these observations, GABA application depolarized the nociceptive afferent and hyperpolarized the non-nociceptive afferent.
Conclusions: These findings show that endocannabinoids can differentially modulate nociceptive vs. non-nociceptive synapses and that GABAergic regulation of these synapses plays an important role in determining whether endocannabinoids have a potentiating or depressing effect.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

