Dual-functional abeo-taxane derivatives destabilizing microtubule equilibrium and inhibiting NF-κB activation
- PMID: 23725535
- PMCID: PMC3755589
- DOI: 10.1021/jm400479p
Dual-functional abeo-taxane derivatives destabilizing microtubule equilibrium and inhibiting NF-κB activation
Abstract
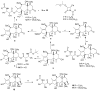
Taxchinin A, with a 11(15→1)-abeo-taxane skeleton, is a major, but inactive taxoid contained in leaves of Taxus chinensis . In our design of dual-functional antitumor abeo-taxane derivatives, two fragments from antitumor agents with different molecular targets (the N-acyl-3'-phenylisoserine side chain from the antimitotic agent paclitaxel and an α,β-unsaturated carbonyl system from NF-κB inhibitors) were incorporated into the scaffold of taxchinin A. The resulting compounds displayed broad inhibitory effects against proliferation of tumor cell lines, with notable selectivity toward colon cancer, melanoma, and renal cancer, when evaluated in the NCI-60 human tumor cell line screening panel. On the basis of the NCI-60 assay data, structure-activity relationship (SAR) correlations were elucidated. Mechanistic studies indicated that this new compound type can both destabilize microtubules and inhibit NF-κB activation, thereby inducing tumor cell apoptosis. This first report of the dual-functional taxoid-core compounds thus provides new opportunities for future drug development based on natural axoid scaffolds.
Figures
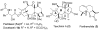






Similar articles
-
Synthesis, cytotoxic activity, and SAR analysis of the derivatives of taxchinin A and brevifoliol.Bioorg Med Chem. 2008 May 1;16(9):4860-71. doi: 10.1016/j.bmc.2008.03.041. Epub 2008 Mar 20. Bioorg Med Chem. 2008. PMID: 18381240 Free PMC article.
-
Synthesis and evaluation of new 2-chloro-4-aminopyrimidine and 2,6-dimethyl-4-aminopyrimidine derivatives as tubulin polymerization inhibitors.Bioorg Med Chem Lett. 2018 Jun 1;28(10):1769-1775. doi: 10.1016/j.bmcl.2018.04.026. Epub 2018 Apr 12. Bioorg Med Chem Lett. 2018. PMID: 29673981
-
Synthesis, anti-cancer evaluation of benzenesulfonamide derivatives as potent tubulin-targeting agents.Eur J Med Chem. 2016 Oct 21;122:488-496. doi: 10.1016/j.ejmech.2016.07.002. Epub 2016 Jul 5. Eur J Med Chem. 2016. PMID: 27423028
-
(Z)-1-aryl-3-arylamino-2-propen-1-ones, highly active stimulators of tubulin polymerization: synthesis, structure-activity relationship (SAR), tubulin polymerization, and cell growth inhibition studies.J Med Chem. 2012 Jun 14;55(11):5174-87. doi: 10.1021/jm300176j. Epub 2012 May 25. J Med Chem. 2012. PMID: 22587519 Free PMC article.
-
Design, synthesis, and biological evaluation of new series of pyrrol-2(3H)-one and pyridazin-3(2H)-one derivatives as tubulin polymerization inhibitors.Bioorg Chem. 2021 Feb;107:104522. doi: 10.1016/j.bioorg.2020.104522. Epub 2020 Dec 1. Bioorg Chem. 2021. PMID: 33317838
Cited by
-
Serotonin type-3 receptor antagonists selectively kill melanoma cells through classical apoptosis, microtubule depolymerisation, ERK activation, and NF-κB downregulation.Cell Biol Toxicol. 2023 Jun;39(3):1119-1135. doi: 10.1007/s10565-021-09667-0. Epub 2021 Oct 15. Cell Biol Toxicol. 2023. PMID: 34654991
-
A One-Pot Three-Component Synthesis and Investigation of the In Vitro Mechanistic Anticancer Activity of Highly Functionalized Spirooxindole-Pyrrolidine Heterocyclic Hybrids.Molecules. 2020 Nov 27;25(23):5581. doi: 10.3390/molecules25235581. Molecules. 2020. PMID: 33261115 Free PMC article.
References
-
- Petrelli A, Giordano S. From single- to multi-target drugs in cancer therapy: when a specificity becomes an advantage. Curr Med Chem. 2008;15:422–432. - PubMed
-
- Morphy R, Rankovic Z. Designing multiple ligands – medicinal chemistry strategies and challenges. Curr Pharm Des. 2009;15:587–600. - PubMed
-
- Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly invitro by taxol. Nature. 1979;277:665–667. - PubMed
-
- Kingston DGI. Taxol, a molecule for all seasons. Chem Commun. 2001:867–880.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

