Hedgehog signaling from the primary cilium to the nucleus: an emerging picture of ciliary localization, trafficking and transduction
- PMID: 23725801
- PMCID: PMC3913210
- DOI: 10.1016/j.gde.2013.04.008
Hedgehog signaling from the primary cilium to the nucleus: an emerging picture of ciliary localization, trafficking and transduction
Abstract
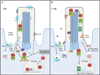
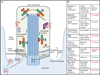
The unexpected connection between cilia and signaling is one of the most exciting developments in cell biology in the past decade. In particular, the Hedgehog (Hh) signaling pathway relies on the primary cilium to regulate tissue patterning and homeostasis in vertebrates. A central question is how ciliary localization and trafficking of Hh pathway components lead to pathway activation and regulation. In this review, we discuss recent studies that reveal the roles of ciliary regulators, components and structures in controlling the movement and signaling of Hh players. These findings significantly increase our mechanistic understanding of how the primary cilium facilitates Hh signal transduction and form the basis for further investigations to define the function of cilia in other signaling processes.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

