Panitumumab and irinotecan versus irinotecan alone for patients with KRAS wild-type, fluorouracil-resistant advanced colorectal cancer (PICCOLO): a prospectively stratified randomised trial
- PMID: 23725851
- PMCID: PMC3699713
- DOI: 10.1016/S1470-2045(13)70163-3
Panitumumab and irinotecan versus irinotecan alone for patients with KRAS wild-type, fluorouracil-resistant advanced colorectal cancer (PICCOLO): a prospectively stratified randomised trial
Abstract
Background: Therapeutic antibodies targeting EGFR have activity in advanced colorectal cancer, but results from clinical trials are inconsistent and the population in which most benefit is derived is uncertain. Our aim was to assess the addition of panitumumab to irinotecan in pretreated advanced colorectal cancer.
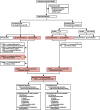
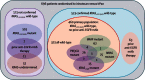
Methods: In this open-label, randomised trial, we enrolled patients who had advanced colorectal cancer progressing after fluoropyrimidine treatment with or without oxaliplatin from 60 centres in the UK. From December, 2006 until June, 2008, molecularly unselected patients were recruited to a three-arm design including irinotecan (control), irinotecan plus ciclosporin, and irinotecan plus panitumumab (IrPan) groups. From June 10, 2008, in response to new data, the trial was amended to a prospectively stratified design, restricting panitumumab randomisation to patients with KRAS wild-type tumours; the results of the comparison between the irinotcan and IrPan groups are reported here. We used a computer-generated randomisation sequence (stratified by previous EGFR targeted therapy and then minimised by centre, WHO performance status, previous oxaliplatin, previous bevacizumab, previous dose modifications, and best previous response) to randomly allocate patients to either irinotecan or IrPan. Patients in both groups received 350 mg/m(2) intravenous irinotecan every 3 weeks (300 mg/m(2) if aged ≥70 years or a performance status of 2); patients in the IrPan group also received intravenous panitumumab 9 mg/kg every 3 weeks. The primary endpoint was overall survival in KRAS wild-type patients who had not received previous EGFR targeted therapy, analysed by intention to treat. Tumour DNA was pyrosequenced for KRASc.146, BRAF, NRAS, and PIK3CA mutations, and predefined molecular subgroups were analysed for interaction with the effect of panitumumab. This study is registered, number ISRCTN93248876.
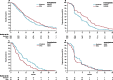
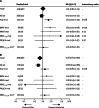
Results: Between Dec 4, 2006, and Aug 31, 2010, 1198 patients were enrolled, of whom 460 were included in the primary population of patients with KRASc.12-13,61 wild-type tumours and no previous EGFR targeted therapy. 230 patients were randomly allocated to irinotecan and 230 to IrPan. There was no difference in overall survival between groups (HR 1·01, 95% CI 0·83-1·23; p=0·91), but individuals in the IrPan group had longer progression-free survival (0·78, 0·64-0·95; p=0·015) and a greater number of responses (79 [34%] patients vs 27 [12%]; p<0·0001) than did individuals in the irinotecan group. Grade 3 or worse diarrhoea (64 [29%] of 219 patients vs 39 [18%] of 218 patients), skin toxicity (41 [19%] vs none), lethargy (45 [21]% vs 24 [11%]), infection (42 [19%] vs 22 [10%]) and haematological toxicity (48 [22%] vs 27 [12%]) were reported more commonly in the IrPan group than in the irinotecan group. We recorded five treatment-related deaths, two in the IrPan group and three in the irinotecan group.
Interpretation: Adding panitumumab to irinotecan did not improve the overall survival of patients with wild-type KRAS tumours. Further refinement of molecular selection is needed for substantial benefits to be derived from EGFR targeting agents.
Funding: Cancer Research UK, Amgen Inc.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

Comment in
-
Will PICCOLO affect metastatic colorectal cancer therapy?Lancet Oncol. 2013 Jul;14(8):679-80. doi: 10.1016/S1470-2045(13)70204-3. Epub 2013 May 29. Lancet Oncol. 2013. PMID: 23725852 No abstract available.
References
-
- Amado RG, Wolf M, Peeters M. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–1634. - PubMed
-
- Van Cutsem E, Kohne CH, Lang I. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011–2019. - PubMed
-
- Bokemeyer C, Bondarenko I, Hartmann JT. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Annals Oncol. 2011;22:1535–1546. - PubMed
-
- Middleton GW, Brown SR, Gwyther SJ. Ciclosporin in combination with irinotecan for chemoresistant advanced colorectal cancer: results of PICCOLO, a large randomised trial with prospective molecular stratification. Eur J Cancer. 2011;47:S420–S421.
-
- Therasse P, Arbuck SG, Eisenhauer EA. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

