Induction of monocyte chemoattractant protein-1 (MCP-1) and its receptor CCR2 in primary sensory neurons contributes to paclitaxel-induced peripheral neuropathy
- PMID: 23726937
- PMCID: PMC3791166
- DOI: 10.1016/j.jpain.2013.03.012
Induction of monocyte chemoattractant protein-1 (MCP-1) and its receptor CCR2 in primary sensory neurons contributes to paclitaxel-induced peripheral neuropathy
Abstract
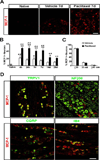
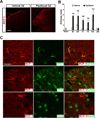
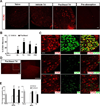
The use of paclitaxel (Taxol), a microtubule stabilizer, for cancer treatment is often limited by its associated peripheral neuropathy (chemotherapy-induced peripheral neuropathy [CIPN]), which predominantly results in sensory dysfunction, including chronic pain. Here we show that paclitaxel CIPN was associated with induction of chemokine monocyte chemoattractant protein-1 (MCP-1) and its cognate receptor CCR2 in primary sensory neurons of dorsal root ganglia. Immunostaining revealed that MCP-1 was mainly expressed in small nociceptive neurons whereas CCR2 was expressed in large and medium-sized myelinated neurons. Direct application of MCP-1 consistently induced intracellular calcium increases in dorsal root ganglia large and medium-sized neurons but not in small neurons mainly dissociated from paclitaxel-treated but not vehicle-treated animals. Paclitaxel also induced increased expression of MCP-1 in spinal astrocytes, but no CCR2 signal was detected in the spinal cord. Local blockade of MCP-1/CCR2 signaling by anti-MCP-1 antibody or CCR2 antisense oligodeoxynucleotides significantly attenuated paclitaxel CIPN phenotypes including mechanical hypersensitivity and loss of intraepidermal nerve fibers in hindpaw glabrous skin. These results suggest that activation of paracrine MCP-1/CCR2 signaling between dorsal root ganglion neurons plays a critical role in the development of paclitaxel CIPN, and targeting MCP-1/CCR2 signaling could be a novel therapeutic approach.
Perspective: CIPN is a severe side effect accompanying paclitaxel chemotherapy and lacks effective treatments. The current study suggests that blocking MCP-1/CCR2 signaling could be a new therapeutic strategy to prevent or reverse paclitaxel CIPN. This preclinical evidence encourages future clinical evaluation of this strategy.
Keywords: CCR2; MCP-1; Paclitaxel; dorsal root ganglion; neuropathy.
Copyright © 2013 American Pain Society. Published by Elsevier Inc. All rights reserved.
Figures
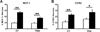


References
-
- Alessandri-Haber N, Yeh JJ, Boyd AE, Parada CA, Chen X, Reichling DB, Levine JD. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron. 2003;39:497–511. - PubMed
-
- Archer DR, Dahlin LB, McLean WG. Changes in slow axonal transport of tubulin induced by local application of colchicine to rabbit vagus nerve. Acta Physiol Scand. 1994;150:57–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

