TIGAR is required for efficient intestinal regeneration and tumorigenesis
- PMID: 23726973
- PMCID: PMC3682186
- DOI: 10.1016/j.devcel.2013.05.001
TIGAR is required for efficient intestinal regeneration and tumorigenesis
Abstract
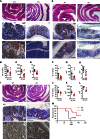
Regulation of metabolic pathways plays an important role in controlling cell growth, proliferation, and survival. TIGAR acts as a fructose-2,6-bisphosphatase, potentially promoting the pentose phosphate pathway to produce NADPH for antioxidant function and ribose-5-phosphate for nucleotide synthesis. The functions of TIGAR were dispensable for normal growth and development in mice but played a key role in allowing intestinal regeneration in vivo and in ex vivo cultures, where growth defects due to lack of TIGAR were rescued by ROS scavengers and nucleosides. In a mouse intestinal adenoma model, TIGAR deficiency decreased tumor burden and increased survival, while elevated expression of TIGAR in human colon tumors suggested that deregulated TIGAR supports cancer progression. Our study demonstrates the importance of TIGAR in regulating metabolism for regeneration and cancer development and identifies TIGAR as a potential therapeutic target in diseases such as ulcerative colitis and intestinal cancer.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures







Comment in
-
Metabolism: a TIGAR tale.Nat Rev Cancer. 2013 Aug;13(8):522. doi: 10.1038/nrc3567. Epub 2013 Jul 4. Nat Rev Cancer. 2013. PMID: 23822981 No abstract available.
References
-
- Barker N., van Es J.H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P.J., Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. - PubMed
-
- Barker N., Ridgway R.A., van Es J.H., van de Wetering M., Begthel H., van den Born M., Danenberg E., Clarke A.R., Sansom O.J., Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. - PubMed
-
- Bensaad K., Vousden K.H. p53: new roles in metabolism. Trends Cell Biol. 2007;17:286–291. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

