Circuit level defects in the developing neocortex of Fragile X mice
- PMID: 23727819
- PMCID: PMC3695061
- DOI: 10.1038/nn.3415
Circuit level defects in the developing neocortex of Fragile X mice
Abstract
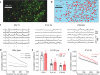
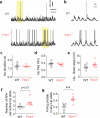
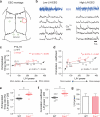
Subtle alterations in how cortical network dynamics are modulated by different behavioral states could disrupt normal brain function and underlie symptoms of neuropsychiatric disorders, including Fragile X syndrome (FXS). Using two-photon calcium imaging and electrophysiology, we recorded spontaneous neuronal ensemble activity in mouse somatosensory cortex. Unanesthetized Fmr1(-/-) mice exhibited abnormally high synchrony of neocortical network activity, especially during the first two postnatal weeks. Neuronal firing rates were threefold higher in Fmr1(-/-) mice than in wild-type mice during whole-cell recordings manifesting Up/Down states (slow-wave sleep, quiet wakefulness), probably as a result of a higher firing probability during Up states. Combined electroencephalography and calcium imaging experiments confirmed that neurons in mutant mice had abnormally high firing and synchrony during sleep. We conclude that cortical networks in FXS are hyperexcitable in a brain state-dependent manner during a critical period for experience-dependent plasticity. These state-dependent network defects could explain the intellectual, sleep and sensory integration dysfunctions associated with FXS.
Figures
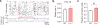

References
-
- Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science. 1996;273:1868–1871. - PubMed
-
- Gilbert CD, Sigman M. Brain states: top-down influences in sensory processing. Neuron. 2007;54:677–696. - PubMed
-
- Crochet S, Petersen CC. Correlating whisker behavior with membrane potential in barrel cortex of awake mice. Nat. Neurosci. 2006;9:608–610. - PubMed
-
- Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat. Neurosci. 2007;10:100–107. - PubMed
-
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

